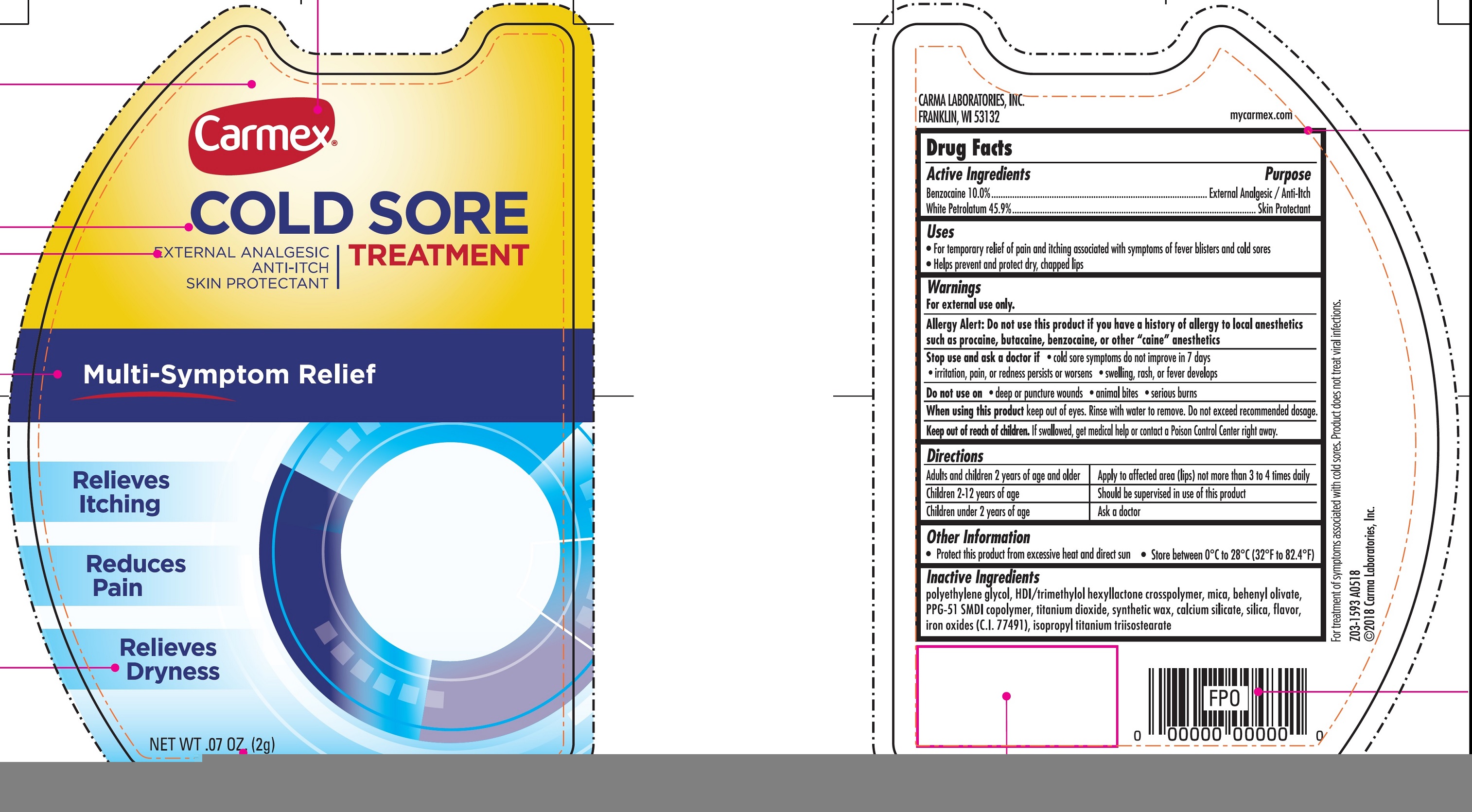
CARMEX COLD SORE treatment External Analgesic Skin Protectant
CARMEX COLD SORE treatment External Analgesic Skin Protectant by
Drug Labeling and Warnings
CARMEX COLD SORE treatment External Analgesic Skin Protectant by is a Otc medication manufactured, distributed, or labeled by Carma Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARMEX COLD SORE TREATMENT EXTERNAL ANALGESIC SKIN PROTECTANT- benzocaine, white petrolatum cream
Carma Laboratories, Inc.
----------
CARMEX COLD SORE treatment External Analgesic Skin Protectant
Uses
- For temporary relief of pain and itching associated with symptoms of fever blisters and cold sores
- Helps prevent and protect dry, chapped lips
Warnings
For external use only.
Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics
Stop use and ask a doctor if
- cold sore symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
Directions
|
Adults and children 2 years of age and older | Apply to affected area (lips) not more than 3 to 4 times daily |
| Children 2-12 years of age | Should be supervised in use of this product |
| Children under 2 years of age | Ask a doctor |
Other Information
- Protect this product from excessive heat and direct sun
- Store between 0°C to 28°C (32°F to 82.4°F)
| CARMEX COLD SORE TREATMENT EXTERNAL ANALGESIC SKIN PROTECTANT
benzocaine, white petrolatum cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Carma Laboratories, Inc. (006090153) |
Revised: 12/2024
Document Id: 29119fd8-49f3-114e-e063-6294a90af6d4
Set id: bada4108-4b3f-8ee6-e053-2995a90a9027
Version: 3
Effective Time: 20241212