MAKE USA- BYHUMANKIND: Kindfill Dental Tabs
Kindfill by
Drug Labeling and Warnings
Kindfill by is a Otc medication manufactured, distributed, or labeled by Make USA LLC, Denttabs GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
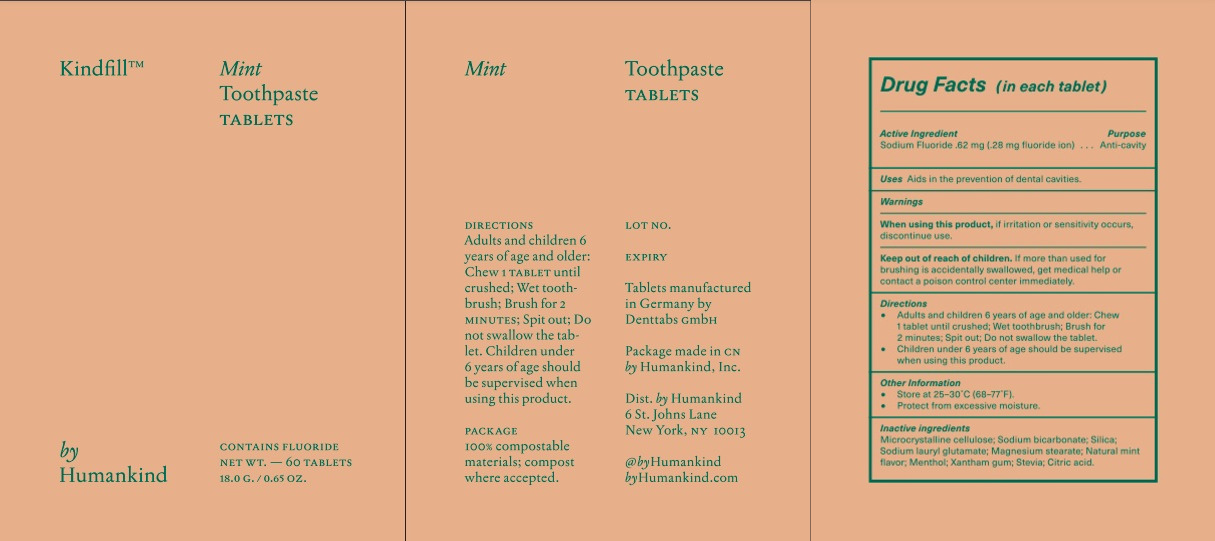
KINDFILL- mint toothpaste tablets tablet, chewable
Make USA LLC
----------
MAKE USA- BYHUMANKIND: Kindfill Dental Tabs
Warnings
- When using this product, if irritation or sensitivity occurs discontinue use.
- Keep out of the reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Adults and children 6 years of age and older; chew one tablet until crushed, wet toothbrush, brush for 2 minutes. Spit out.
- Do not swallow the tablet.
- Children under 6 years of age should be supervised when using this product.
| KINDFILL
mint toothpaste tablets tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Make USA LLC (110142615) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Make USA LLC | 110142615 | manufacture(80486-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denttabs GmbH | 340109625 | manufacture(80486-112) | |
Revised: 10/2025
<
Document Id: 4275dd3f-035c-a927-e063-6294a90acb32
Set id: baeb75d3-c264-118d-e053-2995a90a6208
Version: 3
Effective Time: 20251031
Trademark Results [Kindfill]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KINDFILL 88059393 not registered Live/Pending |
By Humankind, Inc. 2018-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.