ALEVEX- camphor and menthol lotion
AleveX by
Drug Labeling and Warnings
AleveX by is a Otc medication manufactured, distributed, or labeled by Bayer HealthCare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
For external use only
Flammable: Keep away from fire or flame.
When using this product
- avoid contact with the eyes and mucous membranes
- do not bandage tightly
- do not use with a heating pad, medicated patch or other types of local heat
- do not apply to wounds or damaged, broken or irritated skin
- Directions
- Other information
-
Inactive ingredients
aminomethyl propanol, boswellia carterii oil, carbomer interpolymer, clove oil, edetate disodium, eucalyptus oil, fragrance, glycerin, isopulegol, linseed oil, menthoxypropanediol, pentylene glycol, peppermint oil, polysorbate 80, purified water, rosemary oil, SD alcohol 40-B (20.9 % v/v), sorbitan monooleate, thymus mastichina flower oil, tocopherol, vanillyl butyl ether
- Questions or Comments?
- Package label 2.7 oz.
-
INGREDIENTS AND APPEARANCE
ALEVEX
camphor and menthol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0280-0063 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 5.5 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 16 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) THYMUS MASTICHINA FLOWERING TOP OIL (UNII: 9NP0832457) ALCOHOL (UNII: 3K9958V90M) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) CLOVE OIL (UNII: 578389D6D0) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERIN (UNII: PDC6A3C0OX) PEPPERMINT OIL (UNII: AV092KU4JH) FRANKINCENSE OIL (UNII: 67ZYA5T02K) LINSEED OIL (UNII: 84XB4DV00W) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) PENTYLENE GLYCOL (UNII: 50C1307PZG) WATER (UNII: 059QF0KO0R) ROSEMARY OIL (UNII: 8LGU7VM393) VANILLYL BUTYL ETHER (UNII: S2ULN37C9R) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ISOPULEGOL (UNII: 3TH92O3BXN) EDETATE DISODIUM (UNII: 7FLD91C86K) EUCALYPTUS OIL (UNII: 2R04ONI662) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0280-0063-01 77 g in 1 TUBE; Type 0: Not a Combination Product 04/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/01/2021 Labeler - Bayer HealthCare LLC (112117283)
Trademark Results [AleveX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
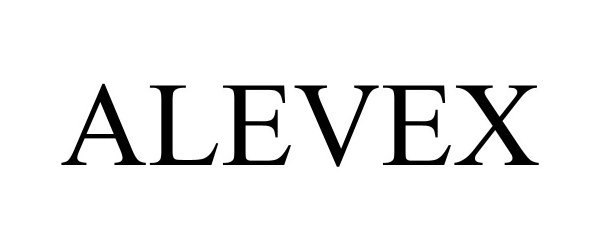 ALEVEX 97865059 not registered Live/Pending |
Bayer HealthCare LLC 2023-03-30 |
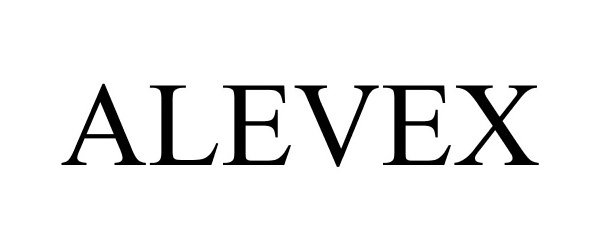 ALEVEX 88656051 not registered Live/Pending |
Bayer HealthCare LLC 2019-10-16 |
 ALEVEX 74292254 not registered Dead/Abandoned |
SYNTEX HEALTH PRODUCTS, INC. 1992-07-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
