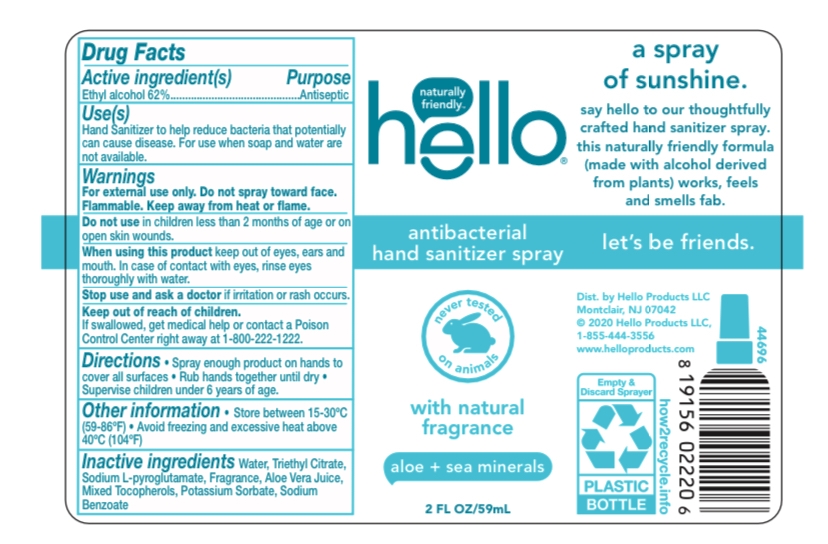
Hello hand sanitizer - aloe and sea minerals
Hello hand sanitizer - aloe and sea minerals by
Drug Labeling and Warnings
Hello hand sanitizer - aloe and sea minerals by is a Otc medication manufactured, distributed, or labeled by Hello Products LLC, MYKU Biosciences LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HELLO HAND SANITIZER - ALOE AND SEA MINERALS- alcohol spray
Hello Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hello hand sanitizer - aloe and sea minerals
Active Ingredient(s) Purpose
Ethyl Alcohol 62%...............................................Antiseptic
Use(s)
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Warnings
For external use only. Do not spray towards face. Flammable. Keep away from heat or flame.
Inactive ingredients
Water, Triethyl Citrate, Sodium L-pyroglutamate, Fragrance, Aloe Vera Juice, Mixed Tocopherols, Potassium Sorbate, Sodium Benzoate
| HELLO HAND SANITIZER - ALOE AND SEA MINERALS
alcohol spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Hello Products LLC (040714890) |
| Registrant - MYKU Biosciences LLC (106728135) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MYKU Biosciences LLC | 106728135 | manufacture(55882-6111) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.