Methyldopa by Mylan Pharmaceuticals Inc. METHYLDOPA tablet
Methyldopa by
Drug Labeling and Warnings
Methyldopa by is a Prescription medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
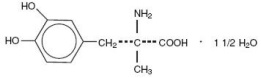
Methyldopa is an antihypertensive and is the L-isomer of alpha-methyldopa. It is levo-3-(3,4-dihydroxyphenyl)-2-methylalanine sesquihydrate. Methyldopa is supplied as tablets for oral administration, containing 250 mg and 500 mg of methyldopa. The amount of methyldopa is calculated on the anhydrous basis. Its molecular formula is C10H13NO41 1/2 H2O, with a molecular weight of 238.24, and its structural formula is:
Methyldopa is a white to yellowish white, odorless fine powder and is sparingly soluble in water.
The tablets contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, sodium lauryl sulfate, titanium dioxide, triacetin, FD&C yellow No. 6 aluminum lake and FD&C blue No. 2 aluminum lake.
-
CLINICAL PHARMACOLOGY
Methyldopa is an aromatic-amino acid decarboxylase inhibitor in animals and in man. Although the mechanism of action has yet to be conclusively demonstrated, the antihypertensive effect of methyldopa probably is due to its metabolism to alpha-methylnorepinephrine, which then lowers arterial pressure by stimulation of central inhibitory alpha-adrenergic receptors, false neurotransmission, and/or reduction of plasma renin activity. Methyldopa has been shown to cause a net reduction in the tissue concentration of serotonin, dopamine, norepinephrine, and epinephrine.
Only methyldopa, the L-isomer of alpha-methyldopa, has the ability to inhibit dopa decarboxylase and to deplete animal tissues of norepinephrine. In man, the antihypertensive activity appears to be due solely to the L-isomer. About twice the dose of the racemate (DL-alpha-methyldopa) is required for equal antihypertensive effect.
Methyldopa has no direct effect on cardiac function and usually does not reduce glomerular filtration rate, renal blood flow, or filtration fraction. Cardiac output usually is maintained without cardiac acceleration. In some patients the heart rate is slowed.
Normal or elevated plasma renin activity may decrease in the course of methyldopa therapy.
Methyldopa reduces both supine and standing blood pressure. It usually produces highly effective lowering of the supine pressure with infrequent symptomatic postural hypotension. Exercise hypotension and diurnal blood pressure variations rarely occur.
Pharmacokinetics and Metabolism
The maximum decrease in blood pressure occurs four to six hours after oral dosage. Once an effective dosage level is attained, a smooth blood pressure response occurs in most patients in 12 to 24 hours. After withdrawal, blood pressure usually returns to pretreatment levels within 24 to 48 hours.
Methyldopa is extensively metabolized. The known urinary metabolites are: α-methyldopa mono-O-sulfate; 3-0-methyl-α-methyldopa; 3,4-dihydroxyphenylacetone; α-methyldopamine; 3-0-methyl-α-methyldopamine and their conjugates.
Approximately 70% of the drug which is absorbed is excreted in the urine as methyldopa and its mono-O-sulfate conjugate. The renal clearance is about 130 mL/min in normal subjects and is diminished in renal insufficiency. The plasma half-life of methyldopa is 105 minutes. After oral doses, excretion is essentially complete in 36 hours.
Methyldopa crosses the placental barrier, appears in cord blood, and appears in breast milk.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Methyldopa is contraindicated in patients:
- with active hepatic disease, such as acute hepatitis and active cirrhosis.
- with liver disorders previously associated with methyldopa therapy (see WARNINGS).
- with hypersensitivity to any component of this product.
- on therapy with monoamine oxidase (MAO) inhibitors.
-
WARNINGS
It is important to recognize that a positive Coombs test, hemolytic anemia, and liver disorders may occur with methyldopa therapy.The rare occurrences of hemolytic anemia or liver disorders could lead to potentially fatal complications unless properly recognized and managed. Read this section carefully to understand these reactions.
With prolonged methyldopa therapy, 10% to 20% of patients develop a positive direct Coombs test which usually occurs between 6 and 12 months of methyldopa therapy. Lowest incidence is at daily dosage of 1 g or less. This on rare occasions may be associated with hemolytic anemia, which could lead to potentially fatal complications. One cannot predict which patients with a positive direct Coombs test may develop hemolytic anemia.
Prior existence or development of a positive direct Coombs test is not in itself a contraindication to use of methyldopa. If a positive Coombs test develops during methyldopa therapy, the physician should determine whether hemolytic anemia exists and whether the positive Coombs test may be a problem. For example, in addition to a positive direct Coombs test there is less often a positive indirect Coombs test which may interfere with cross matching of blood.
Before treatment is started, it is desirable to do a blood count (hematocrit, hemoglobin, or red cell count) for a baseline or to establish whether there is anemia. Periodic blood counts should be done during therapy to detect hemolytic anemia. It may be useful to do a direct Coombs test before therapy and at 6 and 12 months after the start of therapy.
If Coombs-positive hemolytic anemia occurs, the cause may be methyldopa and the drug should be discontinued. Usually the anemia remits promptly. If not, corticosteroids may be given and other causes of anemia should be considered. If the hemolytic anemia is related to methyldopa, the drug should not be reinstituted.
When methyldopa causes Coombs positivity alone or with hemolytic anemia, the red cell is usually coated with gamma globulin of the lgG (gamma G) class only. The positive Coombs test may not revert to normal until weeks to months after methyldopa is stopped.
Should the need for transfusion arise in a patient receiving methyldopa, both a direct and an indirect Coombs test should be performed. In the absence of hemolytic anemia, usually only the direct Coombs test will be positive. A positive direct Coombs test alone will not interfere with typing or cross matching. If the indirect Coombs test is also positive, problems may arise in the major cross match and the assistance of a hematologist or transfusion expert will be needed.
Occasionally, fever has occurred within the first 3 weeks of methyldopa therapy, associated in some cases with eosinophilia or abnormalities in one or more liver function tests, such as serum alkaline phosphatase, serum transaminases (SGOT, SGPT), bilirubin, and prothrombin time. Jaundice, with or without fever, may occur with onset usually within the first 2 to 3 months of therapy. In some patients the findings are consistent with those of cholestasis. In others the findings are consistent with hepatitis and hepatocellular injury.
Rarely, fatal hepatic necrosis has been reported after use of methyldopa. These hepatic changes may represent hypersensitivity reactions. Periodic determinations of hepatic function should be done particularly during the first 6 to 12 weeks of therapy or whenever an unexplained fever occurs. If fever, abnormalities in liver function tests, or jaundice appear, stop therapy with methyldopa. If caused by methyldopa, the temperature and abnormalities in liver function characteristically have reverted to normal when the drug was discontinued. Methyldopa should not be reinstituted in such patients.
Rarely, a reversible reduction of the white blood cell count with a primary effect on the granulocytes has been seen. The granulocyte count returned promptly to normal on discontinuance of the drug. Rare cases of granulocytopenia have been reported. In each instance, upon stopping the drug, the white cell count returned to normal. Reversible thrombocytopenia has occurred rarely.
-
PRECAUTIONS
General
Methyldopa should be used with caution in patients with a history of previous liver disease or dysfunction (see WARNINGS).
Some patients taking methyldopa experience clinical edema or weight gain which may be controlled by use of a diuretic. Methyldopa should not be continued if edema progresses or signs of heart failure appear.
Hypertension has recurred occasionally after dialysis in patients given methyldopa because the drug is removed by this procedure.
Rarely, involuntary choreoathetotic movements have been observed during therapy with methyldopa in patients with severe bilateral cerebrovascular disease. Should these movements occur, stop therapy.
Laboratory Tests
Blood count, Coombs test and liver function tests are recommended before initiating therapy and at periodic intervals (see WARNINGS).
Drug Interactions
When methyldopa is used with other antihypertensive drugs, potentiation of antihypertensive effect may occur. Patients should be followed carefully to detect side reactions or unusual manifestations of drug idiosyncrasy.
Patients may require reduced doses of anesthetics when on methyldopa. If hypotension does occur during anesthesia, it usually can be controlled by vasopressors. The adrenergic receptors remain sensitive during treatment with methyldopa.
When methyldopa and lithium are given concomitantly, the patient should be carefully monitored for symptoms of lithium toxicity. Read the circular for lithium preparations.
Several studies demonstrate a decrease in the bioavailability of methyldopa when it is ingested with ferrous sulfate or ferrous gluconate. This may adversely affect blood pressure control in patients treated with methyldopa. Coadministration of methyldopa with ferrous sulfate or ferrous gluconate is not recommended.
Monoamine oxidase (MAO) inhibitors: See CONTRAINDICATIONS.
Drug/Laboratory Test Interactions
Methyldopa may interfere with measurement of: urinary uric acid by the phosphotungstate method, serum creatinine by the alkaline picrate method, and SGOT by colorimetric methods. Interference with spectrophotometric methods for SGOT analysis has not been reported.
Since methyldopa causes fluorescence in urine samples at the same wave lengths as catecholamines, falsely high levels of urinary catecholamines may be reported. This will interfere with the diagnosis of pheochromocytoma. It is important to recognize this phenomenon before a patient with a possible pheochromocytoma is subjected to surgery. Methyldopa does not interfere with measurement of VMA (vanillylmandelic acid), a test for pheochromocytoma, by those methods which convert VMA to vanillin. Methyldopa is not recommended for the treatment of patients with pheochromocytoma. Rarely, when urine is exposed to air after voiding, it may darken because of breakdown of methyldopa or its metabolites.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a tumorigenic effect was seen when methyldopa was given for 2 years to mice at doses up to 1800 mg/kg/day or to rats at doses up to 240 mg/kg/day (30 and 4 times the maximum recommended human dose in mice and rats, respectively, when compared on the basis of body weight; 2.5 and 0.6 times the maximum recommended human dose in mice and rats, respectively, when compared on the basis of body surface area; calculations assume a patient weight of 50 kg).
Methyldopa was not mutagenic in the Ames Test and did not increase chromosomal aberration or sister chromatid exchanges in Chinese hamster ovary cells. These in vitro studies were carried out both with and without exogenous metabolic activation.
Fertility was unaffected when methyldopa was given to male and female rats at 100 mg/kg/day (1.7 times the maximum daily human dose when compared on the basis of body weight; 0.2 times the maximum daily human dose when compared on the basis of body surface area). Methyldopa decreased sperm count, sperm motility, the number of late spermatids and the male fertility index when given to male rats at 200 and 400 mg/kg/day (3.3 and 6.7 times the maximum daily human dose when compared on the basis of body weight; 0.5 and 1 times the maximum daily human dose when compared on the basis of body surface area).
Pregnancy
Teratogenic Effects. Pregnancy Category B
Reproduction studies performed with methyldopa at oral doses up to 1000 mg/kg in mice, 200 mg/kg in rabbits and 100 mg/kg in rats revealed no evidence of harm to the fetus. These doses are 16.6 times, 3.3 times and 1.7 times, respectively, the maximum daily human dose when compared on the basis of body weight; 1.4 times, 1.1 times and 0.2 times, respectively, when compared on the basis of body surface area; calculations assume a patient weight of 50 kg. There are, however, no adequate and well-controlled studies in pregnant women in the first trimester of pregnancy. Because animal reproduction studies are not always predictive of human response, methyldopa should be used during pregnancy only if clearly needed.
Published reports of the use of methyldopa during all trimesters indicate that if this drug is used during pregnancy the possibility of fetal harm appears remote. In five studies, three of which were controlled, involving 332 pregnant hypertensive women, treatment with methyldopa was associated with an improved fetal outcome. The majority of these women were in the third trimester when methyldopa therapy was begun.
In one study, women who had begun methyldopa treatment between weeks 16 and 20 of pregnancy gave birth to infants whose average head circumference was reduced by a small amount (34.2 ± 1.7 cm vs. 34.6 ± 1.3 cm [mean ± 1 S.D.]). Long-term follow-up of 195 (97.5%) of the children born to methyldopa-treated pregnant women (including those who began treatment between weeks 16 and 20) failed to uncover any significant adverse effect on the children. At 4 years of age, the developmental delay commonly seen in children born to hypertensive mothers was less evident in those whose mothers were treated with methyldopa during pregnancy than those whose mothers were untreated. The children of the treated group scored consistently higher than the children of the untreated group on five major indices of intellectual and motor development. At age 7 and one-half, developmental scores and intelligence indices showed no significant differences in children of treated or untreated hypertensive women.
Nursing Mothers
Methyldopa appears in breast milk. Therefore, caution should be exercised when methyldopa is given to a nursing woman.
Pediatric Use
There are no well-controlled clinical trials in pediatric patients. Information on dosing in pediatric patients is supported by evidence from published literature regarding the treatment of hypertension in pediatric patients. (See DOSAGE AND ADMINISTRATION.)
Geriatric Use
Of the total number of subjects (1,685) in clinical studies of methyldopa, 223 patients were 65 years of age and over, while 33 patients were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. (See DOSAGE AND ADMINISTRATION.)
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Sedation, usually transient, may occur during the initial period of therapy or whenever the dose is increased. Headache, asthenia, or weakness may be noted as early and transient symptoms. However, significant adverse effects due to methyldopa have been infrequent and this agent usually is well tolerated.
The following adverse reactions have been reported and, within each category, are listed in order of decreasing severity.
Cardiovascular: Aggravation of angina pectoris, congestive heart failure, prolonged carotid sinus hypersensitivity, orthostatic hypotension (decrease daily dosage), edema or weight gain, bradycardia.
Digestive: Pancreatitis, colitis, vomiting, diarrhea, sialadenitis, sore or "black" tongue, nausea, constipation, distension, flatus, dryness of mouth.
Endocrine: Hyperprolactinemia.
Hematologic: Bone marrow depression, leukopenia, granulocytopenia, thrombocytopenia, hemolytic anemia; positive tests for antinuclear antibody, LE cells, and rheumatoid factor, positive Coombs test.
Hepatic: Liver disorders including hepatitis, jaundice, abnormal liver function tests (see WARNINGS).
Hypersensitivity: Myocarditis, pericarditis, vasculitis, lupus-like syndrome, drug-related fever, eosinophilia.
Nervous System/Psychiatric: Parkinsonism, Bell's palsy, decreased mental acuity, involuntary choreoathetotic movements, symptoms of cerebrovascular insufficiency, psychic disturbances including nightmares and reversible mild psychoses or depression, headache, sedation, asthenia or weakness, dizziness, light-headedness, paresthesias.
Metabolic: Rise in BUN.
Musculoskeletal: Arthralgia, with or without joint swelling; myalgia.
Respiratory: Nasal stuffiness.
Skin: Toxic epidermal necrolysis, rash.
Urogenital: Amenorrhea, breast enlargement, gynecomastia, lactation, impotence, decreased libido.
-
OVERDOSAGE
Acute overdosage may produce acute hypotension with other responses attributable to brain and gastrointestinal malfunction (excessive sedation, weakness, bradycardia, dizziness, light-headedness, constipation, distention, flatus, diarrhea, nausea, vomiting).
In the event of overdosage, symptomatic and supportive measures should be employed. When ingestion is recent, gastric lavage or emesis may reduce absorption. When ingestion has been earlier, infusions may be helpful to promote urinary excretion. Otherwise, management includes special attention to cardiac rate and output, blood volume, electrolyte balance, paralytic ileus, urinary function and cerebral activity.
Sympathomimetic drugs [e.g., levarterenol, epinephrine, ARAMINE®1 (Metaraminol Bitartrate)] may be indicated. Methyldopa is dialyzable.
The oral LD50 of methyldopa is greater than 1.5 g/kg in both the mouse and the rat.
- 1 The brands listed are trademarks of their respective owners.
-
DOSAGE AND ADMINISTRATION
Adults
Initiation of Therapy
The usual starting dosage of methyldopa tablets is 250 mg two to three times a day in the first 48 hours. The daily dosage then may be increased or decreased, preferably at intervals of not less than 2 days, until an adequate response is achieved. To minimize the sedation, start dosage increases in the evening. By adjustment of dosage, morning hypotension may be prevented without sacrificing control of afternoon blood pressure.
When methyldopa tablets are given to patients on other antihypertensives, the dose of these agents may need to be adjusted to effect a smooth transition. When methyldopa tablets are given with anti-hypertensives other than thiazides, the initial dosage of methyldopa tablets should be limited to 500 mg daily in divided doses; when methyldopa tablets are added to a thiazide, the dosage of thiazide need not be changed.
Maintenance of Therapy
The usual daily dosage of methyldopa tablets is 500 mg to 2 g in two to four doses. Although occasional patients have responded to higher doses, the maximum recommended daily dosage is 3 g. Once an effective dosage range is attained, a smooth blood pressure response occurs in most patients in 12 to 24 hours. Since methyldopa has a relatively short duration of action, withdrawal is followed by return of hypertension usually within 48 hours. This is not complicated by an overshoot of blood pressure.
Occasionally tolerance may occur, usually between the second and third month of therapy. Adding a diuretic or increasing the dosage of methyldopa frequently will restore effective control of blood pressure. A thiazide may be added at any time during methyldopa therapy and is recommended if therapy has not been started with a thiazide or if effective control of blood pressure cannot be maintained on 2 g of methyldopa daily.
Methyldopa is largely excreted by the kidney and patients with impaired renal function may respond to smaller doses. Syncope in older patients may be related to an increased sensitivity and advanced arteriosclerotic vascular disease. This may be avoided by lower doses.
Pediatric Patients
Initial dosage is based on 10 mg/kg of body weight daily in two to four doses. The daily dosage then is increased or decreased until an adequate response is achieved. The maximum dosage is 65 mg/kg or 3 g daily, whichever is less. (See PRECAUTIONS: Pediatric Use.)
-
HOW SUPPLIED
Methyldopa Tablets, USP are supplied as film-coated tablets containing either 250 mg or 500 mg of Methyldopa, USP.
The 250 mg tablets are beige film-coated, round, unscored tablets debossed with MYLAN on one side of the tablet and 611 on the other side. They are available as follows:
NDC: 0378-0611-01
bottles of 100 tabletsThe 500 mg tablets are beige film-coated, capsule-shaped, unscored tablets debossed with MYLAN on one side of the tablet and 421 on the other side. They are available as follows:
NDC: 0378-0421-01
bottles of 100 tabletsStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.REVISED MAY 2015
MD:R14AQ -
PRINCIPAL DISPLAY PANEL - 250 mg
NDC: 0378-0611-01
Methyldopa
Tablets, USP
250 mgRx only 100 Tablets
Each film-coated tablet contains:
Methyldopa (calculated
as anhydrous) 250 mgDispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication
out of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Protect from light.
Usual Adult Dosage: See
accompanying prescribing
information.Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Mylan.com
RM0611A11
-
PRINCIPAL DISPLAY PANEL - 500 mg
NDC: 0378-0421-01
Methyldopa
Tablets, USP
500 mgRx only 100 Tablets
Each film-coated tablet contains:
Methyldopa (calculated
as anhydrous) 500 mgDispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication
out of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Protect from light.
Usual Adult Dosage: See
accompanying prescribing
information.Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Mylan.com
RM0421A12
-
INGREDIENTS AND APPEARANCE
METHYLDOPA
methyldopa tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0378-0611 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLDOPA (UNII: 56LH93261Y) (METHYLDOPA ANHYDROUS - UNII:M4R0H12F6M) METHYLDOPA ANHYDROUS 250 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color BROWN (beige) Score no score Shape ROUND Size 11mm Flavor Imprint Code MYLAN;611 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-0611-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/18/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070076 04/18/1985 METHYLDOPA
methyldopa tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0378-0421 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLDOPA (UNII: 56LH93261Y) (METHYLDOPA ANHYDROUS - UNII:M4R0H12F6M) METHYLDOPA ANHYDROUS 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color BROWN (beige) Score no score Shape OVAL (capsule shaped) Size 19mm Flavor Imprint Code MYLAN;421 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-0421-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/18/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070076 04/18/1985 Labeler - Mylan Pharmaceuticals Inc. (059295980)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.