TYLENOL EXTRA STRENGTH- acetaminophen tablet, coated
TYLENOL by
Drug Labeling and Warnings
TYLENOL by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
children under 12 years ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
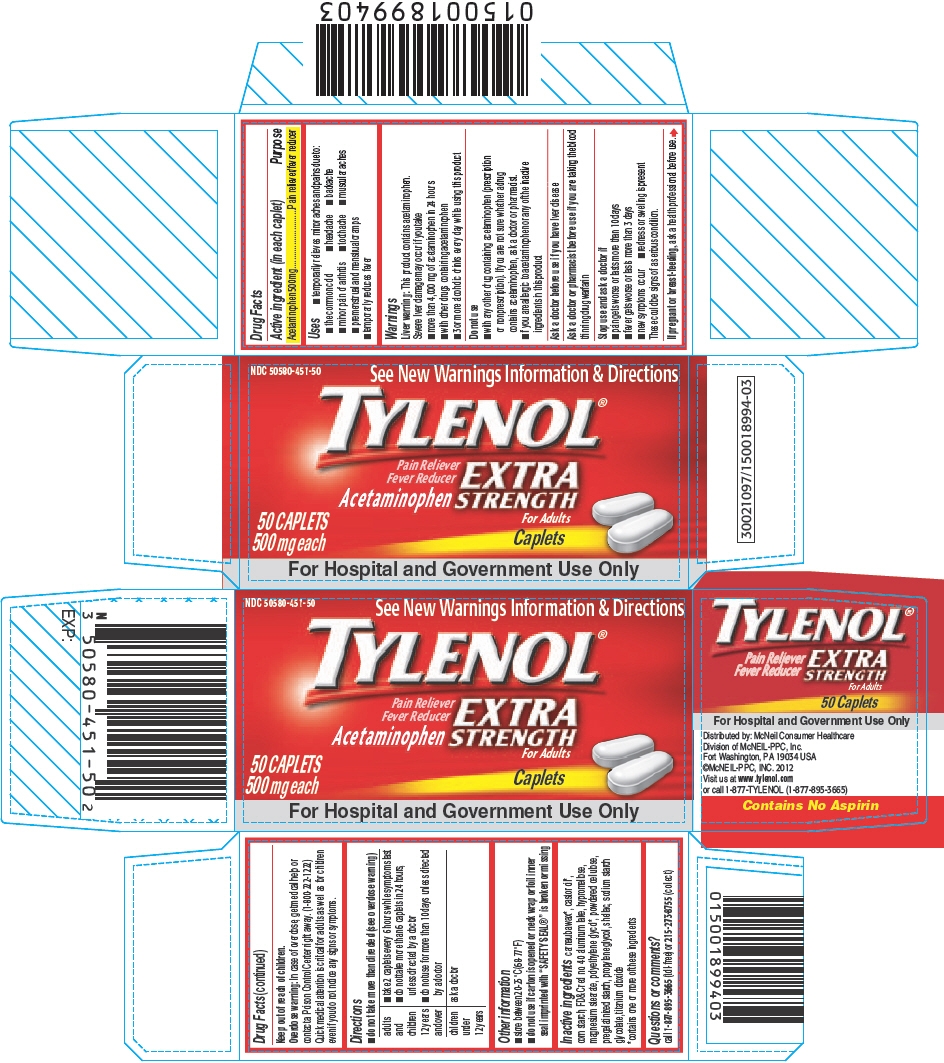
PRINCIPAL DISPLAY PANEL
NDC: 50580-451-50
See New Warnings Information & Directions
TYLENOL®
Pain Reliever
Fever Reducer
AcetaminophenEXTRA
STRENGTH
For Adults50 CAPLETS
500 mg eachCaplets
For Hospital and Government Use Only
-
INGREDIENTS AND APPEARANCE
TYLENOL EXTRA STRENGTH
acetaminophen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50580-451 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 500 mg Inactive Ingredients Ingredient Name Strength Carnauba wax (UNII: R12CBM0EIZ) castor oil (UNII: D5340Y2I9G) starch, corn (UNII: O8232NY3SJ) FD&C red No. 40 (UNII: WZB9127XOA) aluminum oxide (UNII: LMI26O6933) hypromellose, unspecified (UNII: 3NXW29V3WO) magnesium stearate (UNII: 70097M6I30) Polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) powdered cellulose (UNII: SMD1X3XO9M) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Sodium Starch Glycolate Type A Potato (UNII: 5856J3G2A2) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 18mm Flavor Imprint Code TYLENOL;500;HOSPITAL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50580-451-03 150 in 1 CARTON 08/19/1994 11/30/2016 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC: 50580-451-10 10 in 1 CARTON 06/30/2014 01/31/2019 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 50580-451-50 1 in 1 CARTON 08/19/1994 3 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC: 50580-451-70 700 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/19/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 08/19/1994 Labeler - Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division (878046358)
Trademark Results [TYLENOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TYLENOL 98777610 not registered Live/Pending |
Kenvue Inc. 2024-09-30 |
 TYLENOL 97348733 not registered Live/Pending |
Johnson & Johnson 2022-04-06 |
 TYLENOL 90512746 not registered Live/Pending |
Johnson & Johnson 2021-02-05 |
 TYLENOL 88182835 not registered Dead/Abandoned |
Johnson & Johnson 2018-11-06 |
 TYLENOL 85962420 4874809 Live/Registered |
Johnson & Johnson 2013-06-18 |
 TYLENOL 77564801 4096488 Live/Registered |
JOHNSON & JOHNSON 2008-09-08 |
 TYLENOL 77563361 not registered Dead/Abandoned |
Johnson & Johnson 2008-09-05 |
 TYLENOL 76072484 2660253 Live/Registered |
JOHNSON & JOHNSON 2000-06-16 |
 TYLENOL 73824422 1621973 Live/Registered |
JOHNSON & JOHNSON 1989-09-11 |
 TYLENOL 72326412 0890360 Live/Registered |
MCNEIL LABORATORIES, INCORPORATED 1969-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.