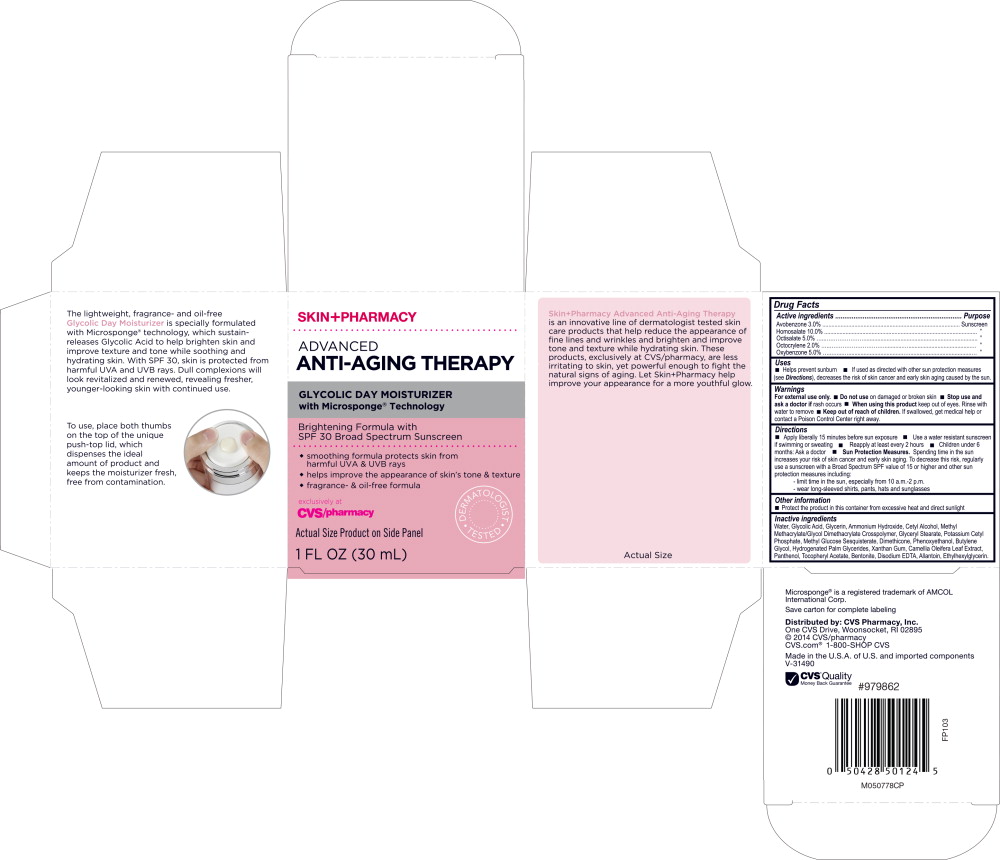
SKINPHARMACY ADVANCED ANTI-AGING THERAPY GLYCOLIC DAY MOISTURIZER SPF 30- homosalate, octisalate, avobenzone, octocrylene, oxybenzone lotion
SkinPharmacy Advanced Anti-Aging Therapy Glycolic Day Moisturizer SPF 30 by
Drug Labeling and Warnings
SkinPharmacy Advanced Anti-Aging Therapy Glycolic Day Moisturizer SPF 30 by is a Otc medication manufactured, distributed, or labeled by AMCOL Health & Beauty Solutions, Inc. DBA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging cause by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- - limit time in the sun, especially from 10 a.m.-2 p.m.
- - wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Water, Glycolic Acid, Glycerin, Ammonium Hydroxide, Cetyl Alcohol, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Glyceryl Stearate, Potassium Cetyl Phosphate, Methyl Glucose Sesquistearate, Dimethicone, Phenoxyethanol, Butylene Glycol, Hydrogenated Palm Glycerides, Xanthan Gum, Camellia Oleifera Leaf Extract, Panthenol, Tocopheryl Acetate, Bentonite, Disodium EDTA, Allantoin, Ethylhexylglycerin.
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and imported components
V-31490
CVS® Quality
Money Back Guarantee
#979862
M050778CP
FP103
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Skin+Pharmacy Advanced Anti-Aging Therapy Carton Label
SKIN+PHARMACY
ADVANCED ANTI-AGING THERAPY
GLYCOLIC DAY MOISTURIZER
with Microsponge® Technology
Brightening Formula with
SPF 30 Broad Spectrum Screen
- smoothing formula protects skin from harmful UVA & UVB rays
- helps improve the appearance of skin's tone & texture
- fragrance- & oil-free formula
exclusively at
CVS/pharmacy
DERMATOLOGIST TESTED
Actual Size Product on Side Panel
1 FL OZ (30 mL)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINPHARMACY ADVANCED ANTI-AGING THERAPY GLYCOLIC DAY MOISTURIZER SPF 30
homosalate, octisalate, avobenzone, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68634-062 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength homosalate (UNII: V06SV4M95S) (homosalate - UNII:V06SV4M95S) homosalate 100 g in 2 mL octisalate (UNII: 4X49Y0596W) (octisalate - UNII:4X49Y0596W) octisalate 50 g in 2 mL avobenzone (UNII: G63QQF2NOX) (avobenzone - UNII:G63QQF2NOX) avobenzone 30 g in 2 mL octocrylene (UNII: 5A68WGF6WM) (octocrylene - UNII:5A68WGF6WM) octocrylene 20 g in 2 mL oxybenzone (UNII: 95OOS7VE0Y) (oxybenzone - UNII:95OOS7VE0Y) oxybenzone 50 g in 2 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) glycolic acid (UNII: 0WT12SX38S) glycerin (UNII: PDC6A3C0OX) Ammonia (UNII: 5138Q19F1X) cetyl alcohol (UNII: 936JST6JCN) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) glyceryl monostearate (UNII: 230OU9XXE4) potassium cetyl phosphate (UNII: 03KCY6P7UT) dimethicone (UNII: 92RU3N3Y1O) Hydrogenated Palm glycerides (UNII: YCZ8EM144Q) allantoin (UNII: 344S277G0Z) butylene glycol (UNII: 3XUS85K0RA) Ethylhexylglycerin (UNII: 147D247K3P) phenoxyethanol (UNII: HIE492ZZ3T) Panthenol (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) Bentonite (UNII: A3N5ZCN45C) Xanthan gum (UNII: TTV12P4NEE) Camellia Oleifera Leaf (UNII: 5077EL0C60) Edetate disodium (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68634-062-01 1 in 1 CARTON 10/01/2014 1 30 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/01/2014 Labeler - AMCOL Health & Beauty Solutions, Inc. DBA (872684803) Establishment Name Address ID/FEI Business Operations AMCOL Health & Beauty Solutions, Inc. DBA 872684803 MANUFACTURE(68634-062) , PACK(68634-062) , LABEL(68634-062) , ANALYSIS(68634-062)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.