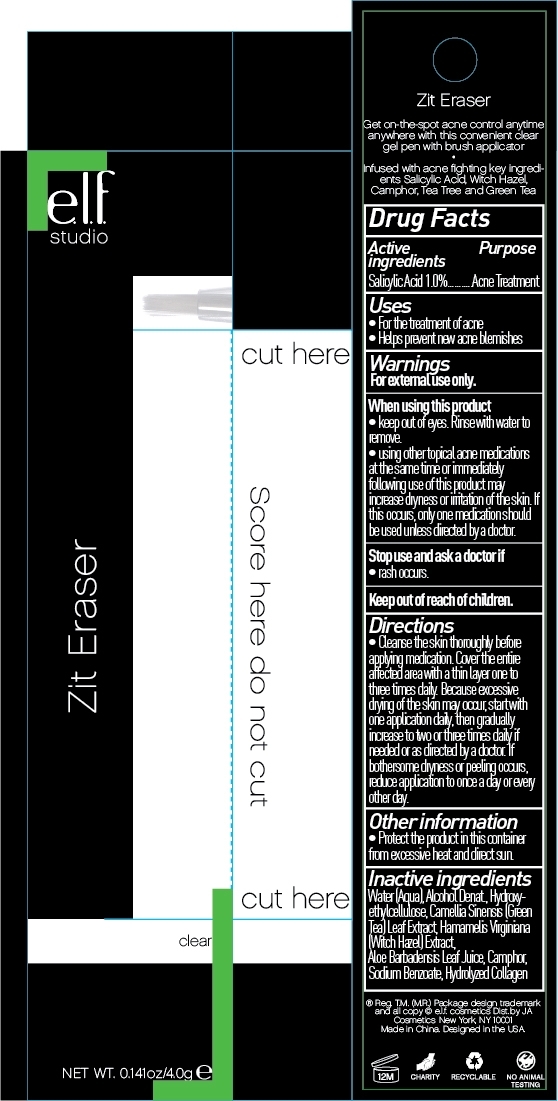
ELF Zit Eraser by J. A. Cosmetics U.S. INC / Shanghai J. A. Cosmetics Trading CO., LTD. Drug Fact
ELF Zit Eraser by
Drug Labeling and Warnings
ELF Zit Eraser by is a Otc medication manufactured, distributed, or labeled by J. A. Cosmetics U.S. INC, Shanghai J. A. Cosmetics Trading CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ELF ZIT ERASER - salicylic acid liquid
J. A. Cosmetics U.S. INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Fact
When Using This Product:
Keep out of eyes, rinse with water to remove.
With other tropical acne medications at the same time or immediately following use of this product may increase dryness or irritation to the skin. If this occurs, only one medication should be used unless directed by your doctor.
Directions:
Cleanse the skin thoroughly before applying medication. Cover the entire affected area with a thin layer one to three times daily. Because excessive drying of skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
| ELF ZIT ERASER
salicylic acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - J. A. Cosmetics U.S. INC (186705047) |