ACETAMINOPHEN PM EXTRA STRENGTH- acetaminophen, diphenhydramine hcl tablet
Acetaminophen PM by
Drug Labeling and Warnings
Acetaminophen PM by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG MARKETING ASSOCIATION INC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each gelcap)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
- Directions
- Other information
-
Inactive ingredients
ammonium hydroxide, colloidal silicon dioxide, croscarmellose sodium, FD&C blue #1, FD&C red #3, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, simethicone, stearic acid, titanium dioxide
- Questions or comments?
-
Principal Display Panel
QC®
QUALITY
CHOICENDC: 63868-210-20
Compare to the active ingredients
in EXTRA STRENGTH
TYLENOL® PM*Extra Strength
Acetaminophen PMAcetaminophen 500 mg,
Diphenhydramine HCl 25 mg
Pain Reliever / Nighttime Sleep-AidRapid Release Gelcaps
20 Gelcaps
Non-Habit Forming
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered
trademark Extra Strength Tylenol® PM.
50844 REV0417B55609Distributed by C.D.M.A., Inc.©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 248-449-9300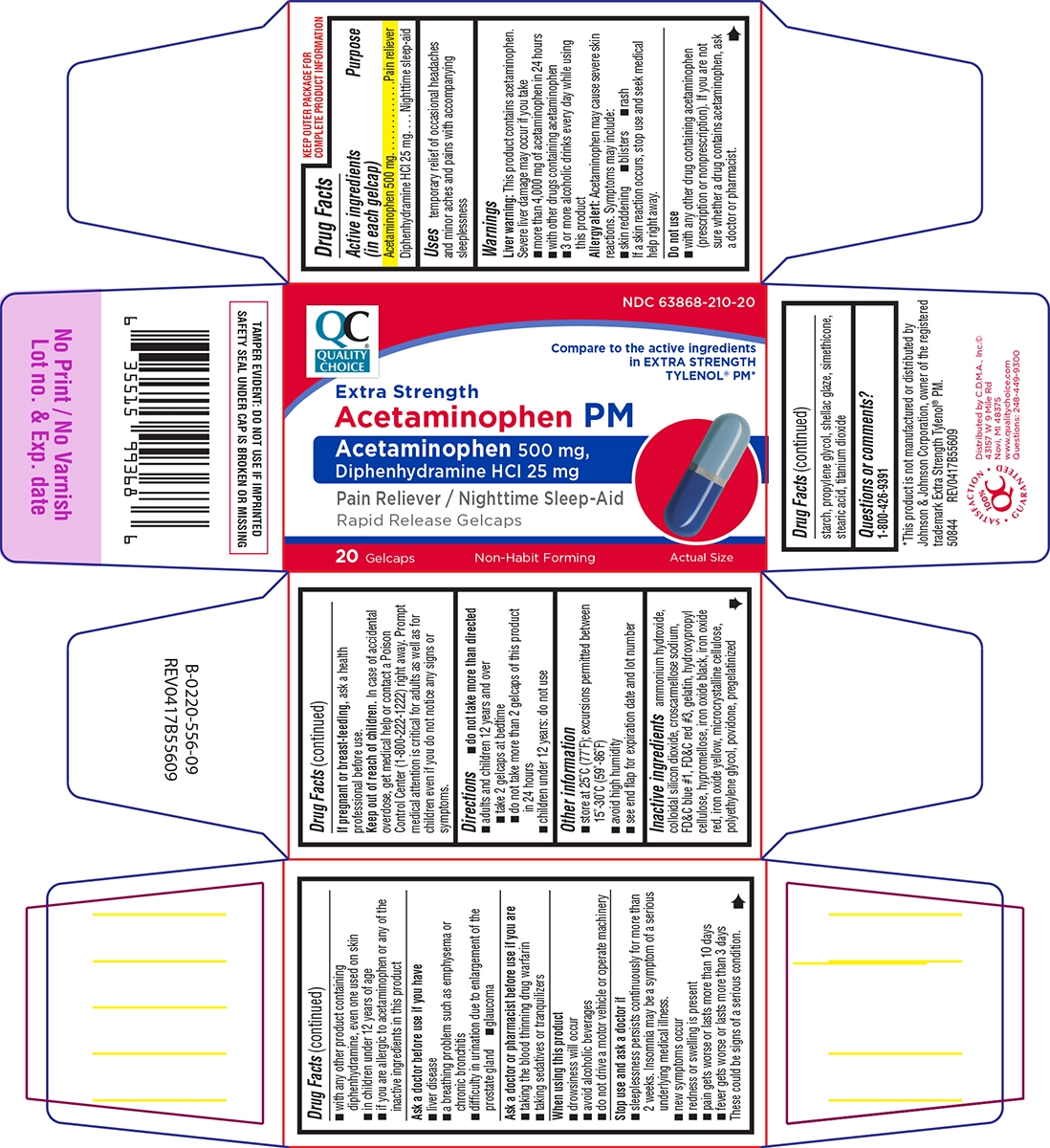
Quality Choice 44-556
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN PM EXTRA STRENGTH
acetaminophen, diphenhydramine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63868-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) AMMONIA (UNII: 5138Q19F1X) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STARCH, CORN (UNII: O8232NY3SJ) SHELLAC (UNII: 46N107B71O) Product Characteristics Color BLUE (dark) , BLUE (light) Score no score Shape OVAL Size 20mm Flavor Imprint Code L;6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63868-210-20 1 in 1 CARTON 12/17/2007 1 20 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 12/17/2007 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(63868-210) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 MANUFACTURE(63868-210) , PACK(63868-210) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(63868-210) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(63868-210)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.