DRAXIMAGE MAA- kit for the preparation of technetium tc 99m albumin aggregated injection, powder, for solution
DRAXIMAGE MAA by
Drug Labeling and Warnings
DRAXIMAGE MAA by is a Prescription medication manufactured, distributed, or labeled by Jubilant DraxImage Inc., Jubilant HollisterStier General Partnership. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
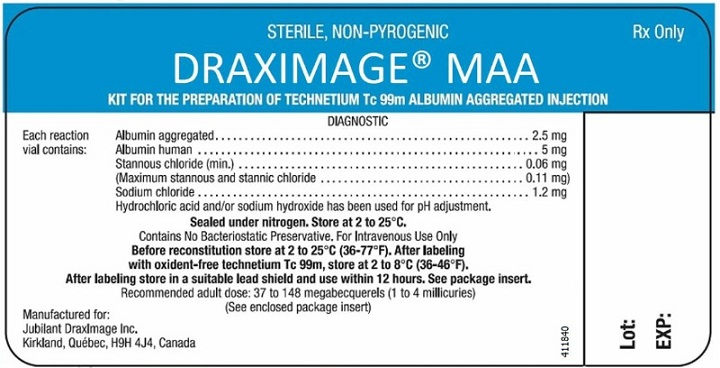
DRAXIMAGE® M AA
Kit for the Preparation ofTechnetium Tc 99mAlbumin Aggregated InjectionDIAGNOSTIC - For Intravenous Use
DESCRIPTION
The kit consists of reaction vials which contain the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Albumin Aggregated Injection for diagnostic use by intravenous injection.
Each 10 mL reaction vial contains 2.5 mg of albumin aggregated, 5 mg of human serum albumin , 0.06 mg (minimum) stannous chloride (maximum stannous and stannic chloride 0.11 mg) and 1.2 mg of sodium chloride; the contents are in a lyophilized form under an atmosphere of nitrogen. Sodium hydroxide or hydrochloric acid has been used for pH adjustment. No bacteriostatic preservative is present.
The human serum albumin was non-reactive when tested for Hepatitis B Surface Antigen (HBsAg), antibodies to Human Immunodeficiency Virus (HIV-1/HIV-2), antibody to Hepatitis C Virus (anti-HCV) and Antigen to Human Immunodeficiency Virus (HIV-1). The aggregated particles are formed by denaturation of the albumin in a heating and aggregation process. Each vial contains 3 to 8 million particles. By light microscopy, more than 90% of the particles are between 10 and 70 micrometers, while the typical average size is 20 to 40 micrometers; none is greater than 150 micrometers.
Technetium Tc 99m Albumin Aggregated Injection for intravenous use is in its final dosage form when a sterile isotonic sodium pertechnetate solution is added to the vial. No less than 90% of the pertechnetate Tc-99m added to a reaction vial is bound to aggregate at preparation time and remains bound throughout the usage lifetime of the preparation (See Directions For Preparation).
PHYSICAL CHARACTERISTICS
Technetium Tc-99m decays by isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging studies is listed in Table 1.
Table 1 - Principal Radiation Emission Data Radiation
Mean %/Disintegration
Mean Energy (keV)
Gamma
89.07
140.5
EXTERNAL RADIATION
The specific gamma ray constant for technetium Tc-99m is 0.78 R/mCi-hr at 1 cm.
The first half value layer is 0.017 cm of lead. A range of values for the relative attenuation of the radiation resulting from the interposition of various thicknesses of lead is shown in Table 2. For example, the use of 0.25 cm thickness of lead will attenuate the radiation emitted by a factor of about 1,000.
Table 2 - Radiation Attenuation by Lead (Pb) Shielding Shield Thickness
(Pb) cm
Coefficient of
Attenuation0.017
0.08
0.16
0.25
0.33
0.5
10-1
10-2
10-3
10-4
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
Immediately following intravenous injection, more than 80% of the albumin aggregated is trapped in the pulmonary alveolar capillary bed. The imaging procedure can thus be started as soon as the injection is complete. Assuming that a sufficient number of radioactive particles has been used, the distribution of radioactive aggregated particles in the normally perfused lung is uniform throughout the vascular bed, and will produce a uniform image. Areas of reduced perfusion will be revealed by a corresponding decreased accumulation of the radioactive particles, and are imaged as areas of reduced photon density.
Organ selectivity is a direct result of particle size. Below 1 to 10 micrometers, the material is taken up by the reticuloendothelial system. Above 10 micrometers, the aggregates become lodged in the lung by a purely mechanical process. Distribution of particles in the lungs is a function of regional pulmonary blood flow.
The albumin aggregated is sufficiently fragile for the capillary micro-occlusion to be temporary. Erosion and fragmentation reduce the particle size, allowing passage of the aggregates through the pulmonary alveolar capillary bed. The fragments are then accumulated by the reticuloendothelial system.
Lung to liver ratios greater than 20:1 are obtained in the first few minutes post-injection. Elimination of the Technetium Tc 99m Aggregated Albumin from the lungs occurs with a half-life of about 2 to 3 hours. Cumulative urinary excretion studies show an average of 20% elimination of the injected technetium Tc 99m dose 24 hours post-administration.
Following administration of Technetium Tc 99m Albumin Aggregated by intraperitoneal injection, the radiopharmaceutical mixes with the peritoneal fluid. Clearance from the peritoneal cavity varies from insignificant, which may occur with complete shunt blockage, to very rapid clearance with subsequent transfer into the systemic circulation when the shunt is patent.
Serial images should be obtained of both the shunt and lung (target organ). However, an adequate evaluation of the difference between total blockage of the shunt and partial blockage may not be feasible in all cases.
-
INDICATIONS AND USAGE
Technetium Tc 99m Albumin Aggregated Injection is a lung imaging agent which may be used as an adjunct in the evaluation of pulmonary perfusion in adults and pediatric patients.
Technetium Tc 99m Albumin Aggregated Injection may be used in adults as an imaging agent to aid in the evaluation of peritoneovenous (LeVeen) shunt patency.
- CONTRAINDICATIONS
-
WARNINGS
Although adverse reactions specifically attributable to Technetium Tc 99m Albumin Aggregated Injection have not been noted, the literature contains reports of deaths occurring after the administration of albumin aggregated to patients with pre-existing severe pulmonary hypertension. Instances of hemodynamic or idiosyncratic reactions to preparations of Technetium Tc 99m Albumin Aggregated have been reported.
-
PRECAUTIONS
General
The contents of the kit before reconstitution are not radioactive. However, after the sodium pertechnetate Tc-99m is added, maintain adequate shielding of the reconstituted product.
In patients with right-to-left heart shunts, additional risk may exist due to the rapid entry of albumin aggregated into the systemic circulation. The safety of this agent in such patients has not been established. Hypersensitivity reactions are possible whenever protein-containing materials such as pertechnetate labeled albumin aggregated are used in man. Epinephrine, antihistamines, and corticosteroids should be available for immediate use.
The intravenous administration of any particulate materials such as albumin aggregated imposes a temporary small mechanical impediment to blood flow. While this effect is probably physiologically insignificant in most patients, the administration of albumin aggregated is possibly hazardous in acute cor pulmonale and other states of severely impaired pulmonary blood flow.
The components of the kit are sterile and non-pyrogenic. It is essential to follow directions carefully and to adhere to strict aseptic procedures during preparation.
Contents of the vials are intended only for use in the preparation of Technetium Tc 99m Albumin Aggregated Injection and are NOT to be administered directly to the patient.
The technetium Tc-99m labeling reactions involved depend on maintaining the stannous ion in the reduced state. Hence, sodium pertechnetate Tc-99m containing oxidants should not be employed.
Technetium Tc 99m Albumin Aggregated Injection is physically unstable and consequently the particles settle with time. Failure to agitate the vial adequately before use may result in nonuniform distribution of radioactive particles.
If blood is drawn into the syringe, unnecessary delay prior to injection may result in clot formation in situ.
Do not use if clumping of the contents is observed.
Technetium Tc 99m Albumin Aggregated Injection, as well as other radioactive drugs, must be handled with care. Once sodium pertechnetate Tc-99m is added to the vial, appropriate safety measures must be used to minimize radiation exposure to clinical personnel. Care must also be taken to minimize the radiation exposure to patients in a manner consistent with proper patient management.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Albumin Aggregated Injection affects fertility in males or females.
Pregnancy Category C
Animal reproduction and teratogenicity studies have not been conducted with Technetium Tc 99m Albumin Aggregated Injection. It is also not known whether Technetium Tc 99m Albumin Aggregated Injection can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. There have been no studies in pregnant women. Technetium Tc 99m Albumin Aggregated Injection should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability, should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Technetium Tc-99m is excreted in human milk during lactation. Therefore, formula feedings should be substituted for breast feedings.
Pediatric Use
The lowest possible number of particles should be used in right-to-left shunting, in neonates, and in severe pulmonary disease.
-
ADVERSE REACTIONS
The literature contains reports of deaths occurring after the administration of albumin aggregated to patients with pre-existing severe pulmonary hypertension. Instances of hemodynamic or idiosyncratic reactions to preparations of Technetium Tc 99m Albumin Aggregated have been reported (see WARNINGS).
-
DOSAGE AND ADMINISTRATION
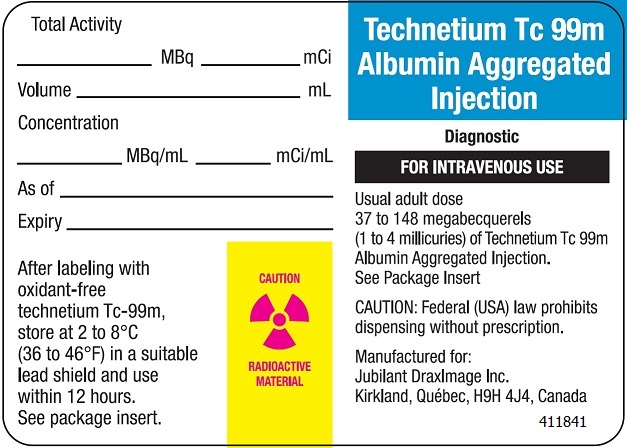
The recommended intravenous dose range for the average (70 kg) ADULT patient for lung imaging is 37 to 148 megabecquerels (1 to 4 millicuries) of Technetium Tc 99m Albumin Aggregated Injection after reconstitution with oxidant-free Sodium Pertechnetate Tc 99m Injection.
The suggested intraperitoneal dosage range used in the average patient (70 kg) for peritoneovenous (LeVeen) shunt patency evaluation is 37 to 111 megabecquerels (1 to 3 millicuries). Adequate measures should be taken to assure uniform mixing with peritoneal fluid. Serial images of both the shunt and target organ should be obtained and correlated with other clinical findings. Alternatively, the drug may be administered by percutaneous transtubal injection. The suggested percutaneous transtubal (efferent limb) dosage range for the average patient (70 kg) is 12 to 37 megabecquerels (0.3 to 1 millicurie) in a volume not to exceed 0.5 mL.
The recommended number of particles per single injection is 200,000 to 700,000 with the suggested number being approximately 350,000. Depending on the activity added and volume of the final reconstituted product, the volume of the dose may vary from 0.2 to 1.9 mL.
The number of particles available per dose of Technetium Tc 99m Albumin Aggregated Injection will vary depending on the physical decay of the technetium Tc-99m that has occurred. The number of particles in any dose and volume to be administered may be calculated as follows:
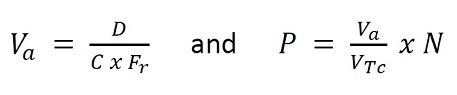
Where:
VTc = volume of solution added to reaction vial
D = desired dose to be administered in MBq (mCi)
C = concentration at calibration time of sodium pertechnetate solution to be added to the reaction vial in MBq/mL (mCi/mL)
Va = volume to be administered in mL
P = number of particles in dose to be administered
Fr = fraction of technetium Tc-99m remaining after the time of calibration (see Table 3)
N = number of particles per vial. The number of particles per vial for each lot is shipped with the product.In PEDIATRIC patients, the suggested intravenous dose to be employed for perfusion lung imaging is in the range of 0.925 to 1.85 MBq per kilogram (25 to 50 μCi/kg) of body weight; a usual dose is 1.11 MBq per kilogram (30 μCi/kg), except in newborns, in whom the administered dose should be 7.4 to 18.5 MBq (200 to 500 μCi). Not less than the minimum dose of 7.4 MBq (200 μCi) should be employed for this procedure. The number of particles will vary with age and weight of the pediatric patient as indicated in Table 5.
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration. Mix the contents of the vial by gentle inversion just prior to withdrawing a patient dose.
Mix the contents of the syringe just before injection. If blood is drawn into the syringe, any unnecessary delay prior to injection may lead to clot formation. For optimum results and because of rapid lung clearance of the radiopharmaceutical, it is suggested that the patient be positioned under the imaging apparatus before administration. Slow injection is recommended. Lung imaging may begin immediately after intravenous injection of the radiopharmaceutical. Due to high kidney uptake, imaging later than one-half hour after administration will yield poor results.
RADIATION DOSIMETRY
The estimated absorbed radiation doses1 to an average ADULT patient (70 kg) from an intravenous injection of 148 MBq (4 mCi) of Technetium Tc 99m Albumin Aggregated Injection are shown in Table 4.
Table 4 - Absorbed Radiation Doses Organs
mGy/148 MBq
rad/4 mCi
Total body
Lungs
Liver
Spleen
Kidneys
Bladder Wall
2 hr. void
4.8 hr. void
Testes
2 hr. void
4.8 hr. void
Ovaries
2 hr. void
4.8 hr. void
0.6
8.8
0.72
0.68
0.44
1.2
2.2
0.24
0.26
0.3
0.34
0.06
0.88
0.072
0.068
0.044
0.12
0.22
0.024
0.026
0.03
0.034
1 Method of calculation: “S” Absorbed Dose per Unit Cumulated Activity for Selected Radionuclides and Organs, MIRD Pamphlet No. 11 (1975). In PEDIATRIC patients, the radiation absorbed doses using the maximum recommended dose for lung imaging are based on 1.85 MBq (50 μCi) per kilogram of body weight [except in the newborn where the maximum recommended dose of 18.5 MBq (500 μCi) is used] and are shown in Table 5, which lists the maximum dose for pediatric patients from newborn to adults. Note the recommendations regarding number of particles to be administered.
Table 6 represents the absorbed radiation dose resulting from the intraperitoneal administration of 111 megabecquerels (3 millicuries) of Technetium Tc 99m Albumin Aggregated.
Table 5 - Pediatric Radiation Dose from Tc 99m MAA for Lung Imaging* (1) 2 hour voiding interval
(2) 4.8 hour voiding intervalAge
Newborn
1 year
5 years
10 years
15 years
Weight (kg)
3.5
12.1
20.3
33.5
55
Max. recommended dose in megabecquerels and millicuries
MBq
18.5mCi
0.5MBq
22.2mCi
0.6MBq
37mCi
1MBq
62.9mCi
1.7MBq
103.6mCi
2.8Range of particles administered
10,000 to
50,00050,000 to
150,000200,000 to
300,000200,000 to
300,000200,000 to
700,000Absorbed radiation dose in milligray and rad for the maximum dose
mGy
rad
mGy
rad
mGy
rad
mGy
rad
mGy
rad
ORGANS
Total body
Lungs
Liver
Bladder wall
Ovaries
Testes
0.6
19
1.4
2.1
0.38
0.31
0.06
1.9
0.14
0.21(1)
0.038
0.031
0.3
6.6
0.6
1.5
0.2
0.13
0.03
0.66
0.06
0.15(1)
0.02
0.013
0.31
5.8
0.62
3.1
0.19
0.19
0.031
0.58
0.062
0.31(2)
0.019
0.019
0.48
8.7
1.8
3.9
0.44
0.2
0.048
0.87
0.18
0.39(2)
0.044
0.02
0.41
7.7
1.2
4.1
0.41
0.36
0.041
0.77
0.12
0.41
0.041
0.036
*Assumptions:
1. Used biologic data from Kaul et al., Berlin, 1973.
2. For the newborn, 1-year old, and 5-year old, the “S” values calculated from the preliminary phantoms of ORNL were used. The 10-year old, 15-year old and adult “S” values were taken from Henrichs et al., Berlin, 1980.Table 6 - Absorbed Radiation Doses* Organs
Shunt Patency
(Open)Shunt Patency
(Closed)mGy
rad
mGy
rad
Lung
Ovaries
& Testes
Organs in the peritoneal cavity
Total body
6.9
0.18
to 0.3
-
0.36
0.69
0.018
to 0.03
-
0.036
1.68
1.68
1.68
0.57
0.168
0.168
0.168
0.057
*Assumptions:
Calculations for the absorbed radiation dose are based upon an effective half-time of 3 hours for the open shunt and 6.02 hours for the closed shunt and an even distribution of the radiopharmaceutical in the peritoneal cavity with no biological clearance. -
HOW SUPPLIED
DRAXIMAGE® MAA (NDC 65174.270.30) comprises:
- 30 multi-dose glass vials. Each vial contains a non-radioactive sterile, non-pyrogenic lyophilized mixture of: 2.5 mg of albumin aggregated, 5 mg of human serum albumin, 0.06 mg (minimum) of stannous chloride (maximum stannous and stannic chloride 0.11 mg), and 1.2 mg of sodium chloride;
- 30 Radiation Labels;
- 1 Package Insert.
HCl and/or NaOH has been used for pH adjustment. The vials are sealed under an atmosphere of nitrogen.
-
STORAGE
The reaction vial contains no bacteriostatic preservative. Store the unreconstituted reaction vials at 2 to 25 ºC (36 to 77 ºF).
After labeling with technetium Tc-99m, store the reconstituted product at 2 to 8 ºC (36 to 46 ºF) when not in use and discard within 12 hours (See Directions For Preparation).
The reconstituted product should be stored during its in-use shelf life in a lead vial shield with cap in place.
-
DIRECTIONS FOR PREPARATION
NOTE: Use aseptic procedures throughout and take precautions to minimize radiation exposure by use of suitable shielding. Waterproof gloves should be worn during the preparation procedure.
Before reconstituting a vial, it should be inspected for cracks and/or a melted plug or any other indication that the integrity of the vacuum seal has been lost.
To prepare Technetium Tc 99m Albumin Aggregated Injection:
1. Remove the protective disc from a reaction vial and swab the rubber septum with either an alcohol swab or a suitable bacteriostatic agent to disinfect the surface.
2. Place the vial in a suitable lead vial shield which has a fitted cap. Obtain 2 to 8 mL of a sterile pyrogen-free Sodium Pertechnetate Tc 99m Injection using a shielded syringe. Sodium pertechnetate Tc 99m solutions containing an oxidizing agent are not suitable for use. The recommended maximum amount of Tc-99m to be added to a reaction vial varies with the number of particles per vial and is shown in Table 7. Other calculations for reconstitution are permitted provided that the patient dose remains within the range prescribed in this package insert (See Dosage and Administration).
Table 7 - Maximum Tc 99m to be Added Based on Vial Particle Number Particles per Vial Maximum Tc 99m to be added per vial* 3 million to 4 million 3.7 GBq (100 mCi) 5 million 4.44 GBq (120 mCi) 6 million to 8 million 6.85 GBq (185 mCi) *Calculate the amount of radioactivity /vial required to maintain the number particles per dose within a recommended range [for adults 200,000 to 700,000, and for pediatric patients follow Table 5].
3. Using a shielded syringe, add the Sodium Pertechnetate Tc 99m Injection to the reaction vial aseptically.
4. Place the lead cap on the vial shield and mix the contents of the shielded vial by repeated gentle inversion until all the material is suspended. Avoid formation of foam. Using proper shielding, the vial should be visually inspected to ensure that the suspension is free of foreign matter before proceeding. Do not administer if foreign particulates are found in the preparation. To ensure maximum tagging, allow the preparation to stand for 15 minutes after mixing.
5. Assay the product in a suitable calibrator, record the radioassay information on the label with radiation warning symbol, and attach it to the vial shield.
6. Withdrawals for administration must be made aseptically using a sterile needle (18 to 21 gauge) and syringe. Since the vials contain nitrogen to prevent oxidation of the complex, the vials should not be vented. If repeated withdrawals are made from the vial, replacement of the contents with air should be minimized.
7. Retain the reconstituted product in the lead vial shield with cap in place during its in-use shelf life. Store the reconstituted product at 2 to 8 ºC (36 to 46 ºF) when not in use and discard within 12 hours depending on the number of particles per vial, the activity added at reconstitution and the final dose to be administered to the patient (See Dosage and Administration).
-
DISPOSAL
The residual materials may be discarded in the ordinary trash, provided the radioactivity in the vials and syringes measures no more than background with an appropriate low-range survey meter. All identifying labels should be destroyed before discarding.
This reagent kit is approved by the U.S. Nuclear Regulatory Commission for distribution to persons licensed to use byproduct material identified in §35.200 of 10 CFR Part 35, to persons who have a similar authorization issued by an Agreement State, and, outside the United States, to persons authorized by the appropriate authority.
Revised: October 2017
Rev. Art: 2.0Jubilant DraxImage Inc.
Kirkland, Québec, H9H 4J4 Canada® Registered Trademark of Jubilant DraxImage Inc.
- MAA Vial Label (Inner Label)
- MAA Carton
- MAA Post-reconstitution Label
-
INGREDIENTS AND APPEARANCE
DRAXIMAGE MAA
kit for the preparation of technetium tc 99m albumin aggregated injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65174-270 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albumin Aggregated (UNII: 799C8VF17R) (Albumin Aggregated - UNII:799C8VF17R) Albumin Aggregated 2.5 mg Inactive Ingredients Ingredient Name Strength Stannous Chloride (UNII: 1BQV3749L5) 0.06 mg ALBUMIN HUMAN (UNII: ZIF514RVZR) 5 mg Sodium Chloride (UNII: 451W47IQ8X) 1.2 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65174-270-30 30 in 1 KIT; Type 1: Convenience Kit of Co-Package 12/30/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA017881 12/30/1987 Labeler - Jubilant DraxImage Inc. (243604761) Registrant - Jubilant DraxImage Inc. (243604761) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier General Partnership 246762764 MANUFACTURE(65174-270)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.