TRIFENA (diclofenac sodium, lidocaine, and menthol,- plusminus patch
Trifena by
Drug Labeling and Warnings
Trifena by is a Otc medication manufactured, distributed, or labeled by Trifluent Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For External Use Only. Topical use only
Allergy Alert
Diclofenac may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- Hives
- Asthma (wheezing)
- Skin Reddening
- Blisters
- Facial Swelling
- Shock
- Rash.
If an allergic reaction occurs, stop use and see medical attention immediately.
Liver Warning
This product contains Diclofenac. Liver damage may occur if you apply
- more or for a longer time than directed or
- when using other drugs containing Diclofenac.
Stomach Bleeding Warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is small, but higher if you:
- Are age 60 or older
- Have had stomach ulcers or bleeding problems
- Taking a blood thinning (anticoagulant) or steroid drug
- Taking other drugs containing prescription or non-prescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- Having 3 or more alcoholic drinks every day.
- Applying more or for longer than directed.
Do Not Use
- On damaged, irritated or infected skin
- With a bandage or eating pad
- If you are allergic to any ingredients in this product
- Right before or after heart surgery
- In the eyes, nose or mouth
- Otherwise than directed.
Stop Use and Ask a Doctor If
- Any of the Warnings apply to you
- You are taking a diurectic
- You are taking any other drugs
- You have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- Excessive skin irritation develops
- Condition worsens
- Symptoms persist for more than 7 days, or symptoms clear up and occur again within 3 days
If Pregnant or Breast-Feeding
Ask a health professional before use.
It is especially important not to use Diclofenac at 20 weeks or later in pregnancy, unless directed by a doctor to do so.
-
Directions
Adults and Children 12 Years of Age and Older:
- Clean and dry to affected area
- Apply gel pad directly to your skin for up to 12 hours
- Using both hands remove the release film from one corner and apply the gel patch adhesive side to the skin affected area.
- Gel pad can be cut into pieces and used on up to 2 body parts at a time
- One application per day is recommended
- Wash hands immediately after use.
- Other Information
-
Inactive Ingredients
Alpha Tocopherol Acetate, Aluminum Glycinate, Aluminium Hydroxide, Borax, Carbomer, Collodial Silicon Dioxide, DMDM Hydantion, Glycerine, Polyacrylic Acid, Polyvinyl Alcohol, Polyvinylpyrrolidone, Propylene Glycol, Purified Water, Sodium Carboxymethyl Cellulose, Sodium Ethylenediaminetetraacetic Acid, Sodium Polyacrylate, Sorbitan Monooleate, Tartaric Acid & Titanium Dioxide.
- Questions or Comments?
-
PRINCIPAL DISPLAY PANEL - 15 Patch Carton
NDC: 73352-565-01
15 PATCHES
trifena™
Pain Relief Patch
Diclofenac Sodium, USP 1.2%
Menthol, USP 5%
Lidocaine, USP 4%TRIFLUENT
PHARMA®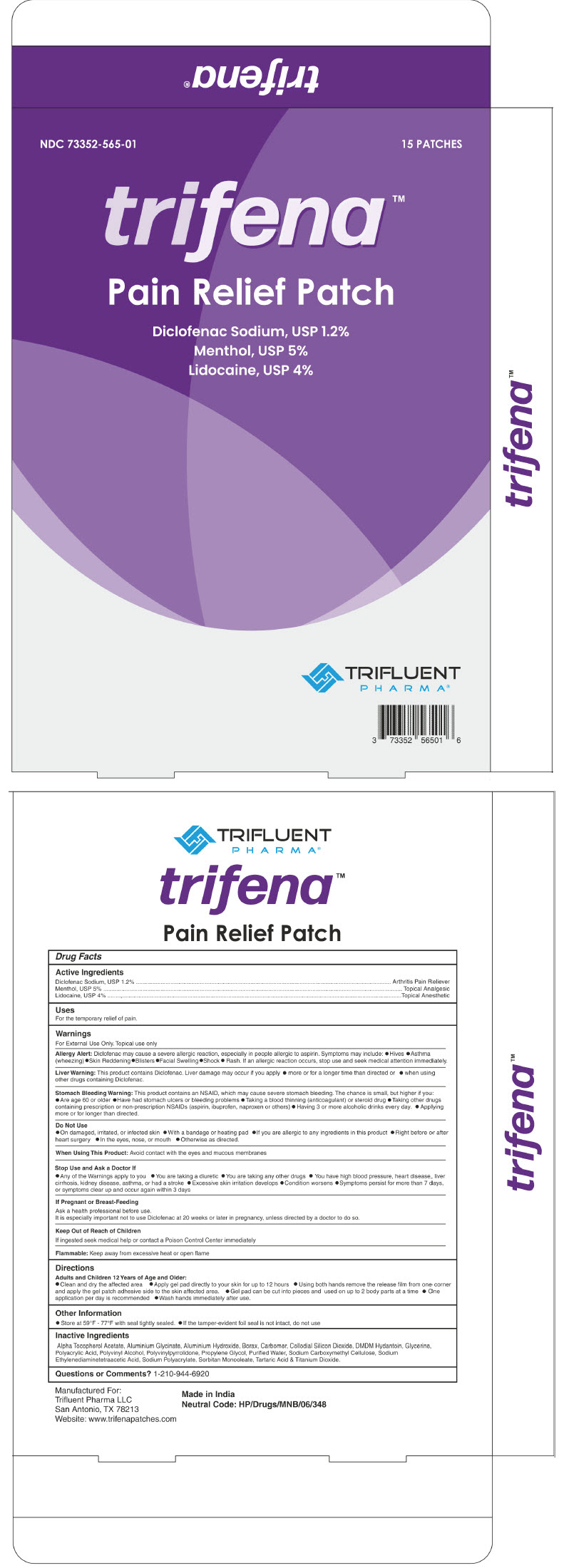
-
INGREDIENTS AND APPEARANCE
TRIFENA
diclofenac sodium, lidocaine, and menthol, (plus)minus patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73352-565 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 0.012 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.04 g MENTHOL, (+)- (UNII: C6B1OE8P3W) (MENTHOL, (+)- - UNII:C6B1OE8P3W) MENTHOL, (+)- 0.05 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM BORATE (UNII: 91MBZ8H3QO) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DMDM HYDANTOIN (UNII: BYR0546TOW) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K90 (UNII: RDH86HJV5Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73352-565-01 15 in 1 CARTON; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 Labeler - Trifluent Pharma LLC (117167281)
Trademark Results [Trifena]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRIFENA 78052893 not registered Dead/Abandoned |
POSITIVE BIOTECH INC. 2001-03-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.