SPF 30 by FRIDDA DORSCH SL / FRIDDA DORSCH
SPF 30 by
Drug Labeling and Warnings
SPF 30 by is a Otc medication manufactured, distributed, or labeled by FRIDDA DORSCH SL, FRIDDA DORSCH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
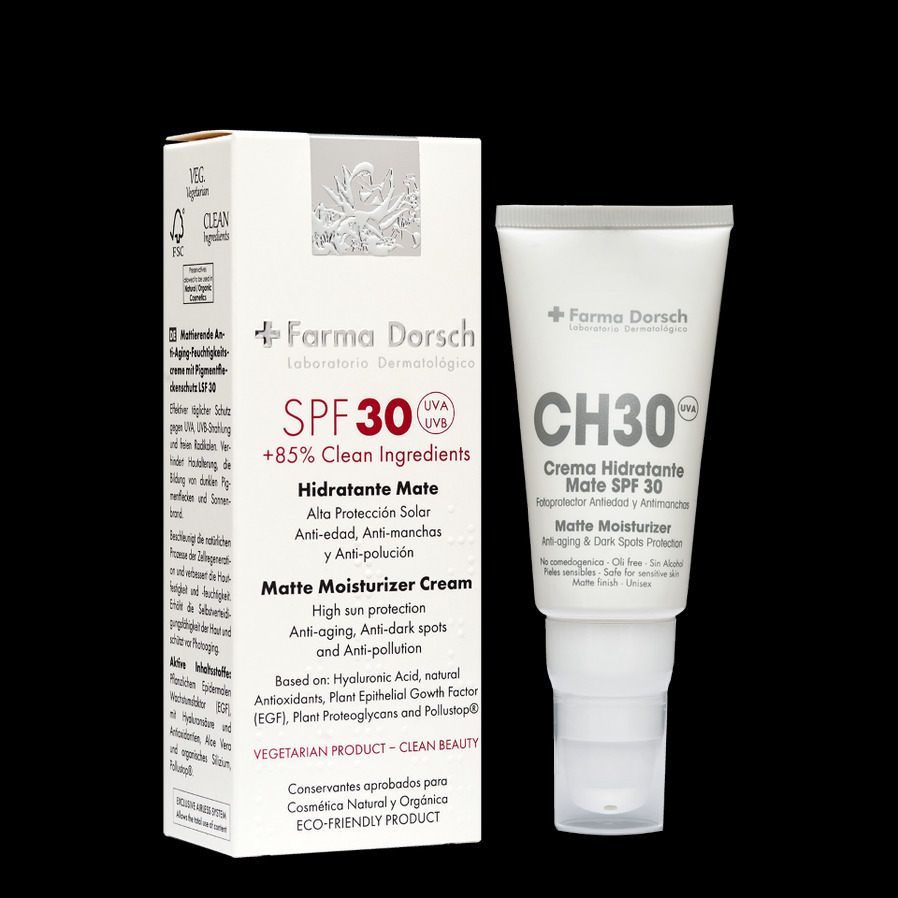
SPF 30- sunscreen cream
FRIDDA DORSCH SL
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SPF 30
Moisturizer Cream with High Protection
Trasparent and Matt looking
Anti-aging , Anti-dark spots and Anti-Pollution
Moisturizing cream with daily protection of SPF 30. Prevents photo-aging, dark spot formation and solar erythema. All skin types, especially suitable for more photosensitive skin. With selected active ingredients such as plant-based Epithelial Growth Factor (EGF), Hyaluronic Acid concentrate, stabilized organic Silicon, plant-derived Proteoglycans, Pollustop®️ and organic Aloe Vera.
1. It guarantees a very effective high daily protection UVA, UVB and free radical radiation
2. Accelerates natural cell regeneration processes
3. Improves the biological balance of cells
4. Increases sensitive or reactive skin self-defense ability
5. Avoid damage caused by the Pollution on the skin
Instruction to use:
1. Apply on dry skin at least 30 minutes before sun exposure
2. Apply to the face until completely absorbed ensuring that the skin is covered by an even layer of product
3. We recommend to wait a couple of minutes until completely absorbed before applying another product on top

SPF 30 UVA
Antiaging Photoprotector
Moisturizer with very high Protection

Moisturizing cream with daily protection of SPF 30. Prevents photo-aging, dark spot formation and solar erythema. All skin types, especially suitable for more photosensitive skin. With selected active ingredients such as plant-based Epithelial Growth Factor (EGF), Hyaluronic Acid concentrate, stabilized organic Silicon, plant-derived Proteoglycans, Pollustop®️ and organic Aloe Vera.
1. It guarantees a very effective high daily protection UVA, UVB and free radical radiation
2. Accelerates natural cell regeneration processes
3. Improves the biological balance of cells
4. Increases sensitive or reactive skin self-defense ability
5. Avoid damage caused by the Pollution on the skin
Instruction to use:
1. Apply on dry skin at least 30 minutes before sun exposure
2. Apply to the face until completely absorbed ensuring that the skin is covered by an even layer of product
3. We recommend to wait a couple of minutes until completely absorbed before applying another product on top


| SPF 30
sunscreen cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - FRIDDA DORSCH SL (474018686) |
| Registrant - FRIDDA DORSCH SL (474018686) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| FRIDDA DORSCH | 474018686 | manufacture(72597-1946) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.