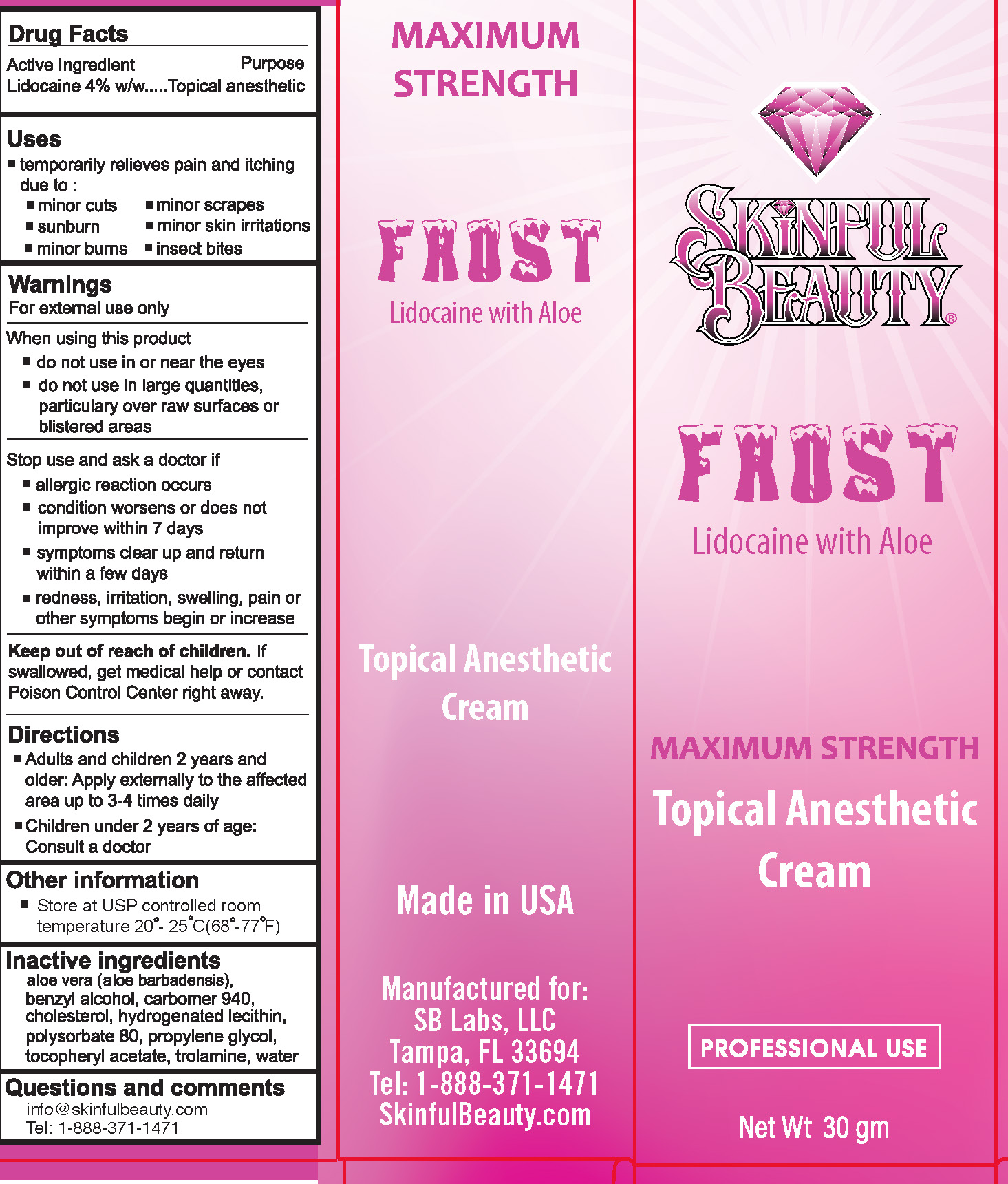
SB Lidocaine 4% with Aloe
Frost Lidocaine with Aloe by
Drug Labeling and Warnings
Frost Lidocaine with Aloe by is a Otc medication manufactured, distributed, or labeled by DSC Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FROST LIDOCAINE WITH ALOE- lidocaine 4% cream
DSC Laboratories Inc.
----------
SB Lidocaine 4% with Aloe
Uses
temporarily relieves pain and itching due to:
- minor cuts
- minor scrapes
- sunburn
- minor skin irritations
- minor burns
- insect bites
WARNINGS
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
Directions
- Adults and children 2 years and older: Apply externally to the affected area up to 3-4 times daily.
- Children under 2 years of age: Consult a doctor.
| FROST LIDOCAINE WITH ALOE
lidocaine 4% cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - DSC Laboratories Inc. (097807374) |
| Registrant - DSC Laboratories Inc. (097807374) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DSC Laboratories Inc. | 097807374 | manufacture(52316-031) | |
Revised: 12/2023
Document Id: 0c00ce56-67e5-c716-e063-6294a90a52eb
Set id: bc1635df-11ad-762e-e053-2995a90a7152
Version: 3
Effective Time: 20231208
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.