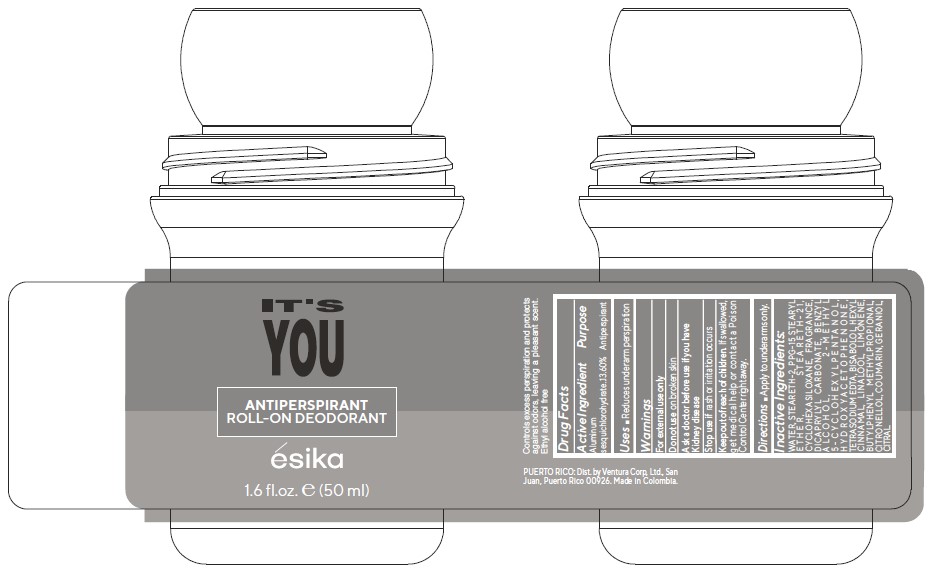
It's you Antiperspirant Roll-on Deodorant
Its you Antiperspirant Roll-on Deodorant by
Drug Labeling and Warnings
Its you Antiperspirant Roll-on Deodorant by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD, BEL STAR S A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ITS YOU ANTIPERSPIRANT ROLL-ON DEODORANT- aluminum sesquichlorohydrate emulsion
Ventura Corporation LTD
----------
It's you Antiperspirant Roll-on Deodorant
Keep out of reach of children.If swallowed, get medical Help or contact a Poison Control Center right away.
Warnings
For external use only
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Inactive ingredients:WATER, STEARETH-2, PPG-15 STEARYL ETHER, STEARETH-21, CYCLOHEXASILOXANE, FRAGRANCE, DICAPRYLYL CARBONATE, BENZYL ALCOHOL, 2-METHYL 5-CYCLOHEXYLPENTANOL, HYDROXYACETOPHENONE, TETRASODIUM EDTA, BISABOLOL, HEXYL CINNAMAL, LINALOOL, LIMONENE, BUTYLPHENYL METHYLPROPIONAL, CITRONELLOL, COUMARIN, GERANIOL, CITRAL.
| ITS YOU ANTIPERSPIRANT ROLL-ON DEODORANT
aluminum sesquichlorohydrate emulsion |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Ventura Corporation LTD (602751344) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BEL STAR S A | 880160197 | manufacture(43596-0200) | |