ZUPREVO- tildipirosin injection, solution
Zuprevo by
Drug Labeling and Warnings
Zuprevo by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Antimicrobial Drug
- CAUTION
-
DESCRIPTION
Zuprevo™18% is a ready-to-use sterile injectable solution containing tildipirosin, a semi-synthetic macrolide antibiotic. Each mL of Zuprevo™18% contains 180 mg of tildipirosin as the free base, 82.5 mg citric acid monohydrate and 400 mg propylene glycol, and water qs with citric acid monohydrate added to adjust pH.
CHEMICAL NOMENCLATURE AND STRUCTURE
Tildipirosin is the nonproprietary name for (11E,13E)-(4R,5S,6S,7R,9R,15R,16R)-6-(4-Dimethylamino-3,5-dihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-16-ethyl-4-hydroxy-5,9,13-trimethyl-7-(2-piperidin-1-yl-ethyl)-15-piperidin-1-ylmethyl-oxacyclohexadeca-11,13-diene-2,10-dione. The empirical formula is C41H71N3O8. The chemical structure of tildipirosin is shown below.
Figure 1
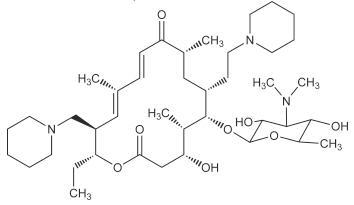
-
INDICATIONS
Zuprevo™18% is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in beef and and non-lactating dairy cattle, and for the control of respiratory disease in beef and non-lactating dairy cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni.
-
DOSAGE AND ADMINISTRATION
Inject subcutaneously as a single dose in the neck at a dosage of 4 mg/kg (1 mL/100 lb) body weight (BW). Do not inject more than 10 mL per injection site. Do not puncture the stopper of the respective vial size more than the tested number of punctures, shown in Table 1.
Clinical field studies indicate that administration of Zuprevo™ 18% (tildipirosin) Injectable Solution is effective for the control of respiratory disease in beef and non-lactating dairy cattle at "high risk" of developing BRD. Calves at high risk of developing BRD typically experience one or more of the following risk factors:
- Commingling from multiple sale barns/sources
- Extended transport times and shrink
- Exposure to wet or cold weather conditions or wide temperature swings
- Stressful arrival processing procedures (such as castration, dehorning, or branding)
- Recent weaning and poor vaccination history
Table 1 Number of punctures tested in the in-use study for the respective vial sizes Vial size [mL] Number of punctures tested in the in-use study 50 8 100 8 250 16 -
WARNINGS
FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. TO AVOID ACCIDENTAL INJECTION, DO NOT USE IN AUTOMATICALLY POWERED SYRINGES WHICH HAVE NO ADDITIONAL PROTECTION SYSTEM. IN CASE OF HUMAN INJECTION, SEEK MEDICAL ADVICE IMMEDIATELY AND SHOW THE PACKAGE INSERT OR LABEL TO THE PHYSICIAN.
Avoid direct contact with skin and eyes. If accidental eye exposure occurs, rinse eyes with clean water. If accidental skin exposure occurs, wash the skin immediately with soap and water. Tildipirosin may cause sensitization by skin contact.
For technical assistance or to report a suspected adverse reaction, call: 1-800-219-9286.
For customer service or to request a Material Safety Data Sheet (MSDS), call: 1-800-211-3573.
For additional Zuprevo™18% information go to www.zuprevo.com.
For a complete listing of adverse reactions for Zuprevo™18% reported to CVM see: http://www.fda.gov/AnimalVeterinary/SafetyHealth.
DO NOT USE ZUPREVO 18% IN SWINE. Fatal adverse events have been reported following the use of tildipirosin in swine. NOT FOR USE IN CHICKENS OR TURKEYS.
-
RESIDUE WARNING
RESIDUE WARNING
Cattle intended for human consumption must not be slaughtered within 21 days of the last treatment. Do not use in female dairy cattle 20 months of age or older. Use of this drug product in these cattle may cause milk residues. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
-
PRECAUTIONS
The effects of Zuprevo™18% on bovine reproductive performance, pregnancy and lactation have not been determined. Swelling and inflammation, which may be severe, may be seen at the injection site after administration. Subcutaneous injection may result in local tissue reactions which persist beyond the slaughter withdrawal period. This may result in trim loss of edible tissue at slaughter.
-
CLINICAL PHARMACOLOGY
Similar to other macrolides, tildipirosin inhibits essential bacterial protein biosynthesis with selective binding to ribosomal subunits in a bacteriostatic and time-dependent manner. Tildipirosin may be bactericidal against certain isolates of Mannheimia haemolytica and Pasteurella multocida.
The following plasma pharmacokinetic (PK) properties of tildipirosin have been observed following a subcutaneous injection at a dose of 4 mg/kg body weight in the neck:
Table 2 Summary of pharmacokinetic characterization of tildipirosin administered subcutaneously to calves at a dose of 4 mg/kg BW. Parameter Average SD Cmax: maximum observed plasma concentration Tmax: Time at which Cmax was observed AUC0-last: Area under the plasma concentration versus time curve measured from time zero to the last sample with tildipirosin concentrations exceeding the limit of quantification of the analytical method AUC0-inf: AUC estimated from time zero to time infinity t1/2: Terminal elimination half life - * Value based on all 14 animals
- † Value based on 8 animals that were slaughtered at 504 hr post-treatment.
Cmax (ng/mL) 767* 284 Tmax (hr) 0.75* 0.43 AUC0-last (hr∙ng/mL) 21017† 3499 AUC0-inf (hr∙ng/mL) 24934† 3508 t1/2 (hr) 210† 53 Due to the extensive partitioning of macrolides into tissues and because of their multi-fold greater concentrations in bronchial fluid relative to that observed in the blood, plasma drug concentrations underestimate concentrations at the site of action1. This is shown for tildipirosin in the following table, where bronchial fluid samples were collected in live, healthy calves, and compared to the concentrations in plasma observed in these same animals:
Table 3 Bronchial fluid-to-plasma ratio of tildipirosin in non-anesthetized cattle following a subcutaneous injection at a dose of 4 mg/kg body weight in the neck Time
(hours)Bronchial fluid (BF)
concentration (ng/g)Plasma (P)
concentration (ng/mL)BF/P
RatioAverage SD Average SD 4 1543 895 297 81.8 5.20 10 2975 1279 242 96.7 12.3 24 3448 1433 136 53.9 25.4 72 3489 1712 70.7 29.0 49.3 96 1644 2024 60.2 29.0 27.3 120 1619 1629 52.3 19.9 30.9 240 1937 1416 27.1 10.8 71.5 336 1225 1682 26.1 9.2 47.0 504 935 1032 16.8 1.7 55.6 Tildipirosin concentrations in bronchial fluid collected in vivo from non-anesthetized cattle reflect the bacterial exposure to drug concentrations at the site of action.
- 1 Nightingale, C.H. (1997) Pharmacokinetics and pharmacodynamics of newer macrolides. The Pediatric Infectious Disease Journal, 16, 438-443.
-
MICROBIOLOGY
Tildipirosin has shown in vitro and in vivo antibacterial activity against the bacteria Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, three pathogens associated with bovine respiratory disease (BRD).
The minimum inhibitory concentrations (MICs) of tildipirosin against the indicated BRD pathogens were determined using the methods described in the M31-A2 standard of the Clinical and Laboratory Standards Institute (CLSI) and are shown in Table 4.
The MICs of tildipirosin were determined for isolates of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni obtained from two BRD field studies. In both studies, tested isolates of M. haemolytica and P. multocida were obtained from nasopharyngeal swabs taken prior to treatment from all study animals. Tested isolates of H. somni were obtained from nasopharyngeal swabs taken prior to treatment from all study animals and from nasopharyngeal swabs taken from saline-treated animals classified as treatment failures.
Table 4 Tildipirosin minimum inhibitory concentration (MIC) values* of indicated pathogens isolated from BRD field studies in the U.S. Indicated Pathogens Year of isolation Study Number of isolates MIC50† (µg/mL) MIC90† (µg/mL) MIC range (µg/mL) - * The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
- † The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
Mannheimia haemolytica 2007 Treatment 484 1 2 0.25 to >32 2007 to 2008 Control 178 1 1 0.25 to >32 Pasteurella multocida 2007 Treatment 235 0.5 1 0.12 to >32 2007 to 2008 Control 273 0.5 1 ≤0.03 to 4 Histophilus somni 2007 Treatment 33 2 4 1 to 4 2007 to 2008 Control 32 2 4 1 to >32 -
EFFECTIVENESS
In a multi-location field study, calves with naturally occurring BRD were treated with tildipirosin. The treatment success rate of the tildipirosin-treated group was compared to the treatment success rate in the saline-treated control group. A treatment success was defined as a calf not designated as a treatment failure from Day 1 to 13 and with normal attitude, normal respiration, and a rectal temperature of < 104°F on Day 14. The treatment success rate was significantly higher (p = 0.003) for the tildipirosintreated group (229/300, 76%) compared to the saline-treated control group (96/200, 32%). There were no BRD-related deaths in the tildipirosin-treated group compared to a 7% (21/300) BRD-related mortality rate in the saline-treated group.
In another multi-location field study, calves at high risk for developing BRD were administered tildipirosin. The treatment success rate of the tildipirosin-treated group was compared to the treatment success rate in the saline-treated control group. A treatment success was defined as a calf not designated as a treatment failure based on clinical respiratory and attitude scoring and, if necessary, rectal temperature measurement of < 104°F through the end of the study (Day 14). The treatment success rate was significantly higher (p = 0.0001) for the tildipirosin-treated group (305/386, 79%) compared to the saline-treated group (197/387, 51%). There were three BRD-related deaths during the study (one tildipirosin-treated calf and two saline treated calves).
-
ANIMAL SAFETY
A target animal safety study was conducted using Zuprevo™18% administered in 5-month-old cattle as three subcutaneous doses of 4, 12, or 20 mg/kg BW given 7 days apart (1×, 3×, and 5× the labeled dose). Animals remained clinically healthy during the study at the labeled dose. Injection site swelling and inflammation, initially severe in some animals, was observed that persisted to the last day of observation (21 days after injection). No other drug-related lesions were observed macroscopically or microscopically at the labeled dose.
A separate injection site tolerance study was conducted using Zuprevo™18% in 5- to 9-month-old cattle administered as a single subcutaneous injection of 10 mL. Injection site swelling and inflammation, initially severe in some animals, was observed that persisted to the last day of observation (35 days after injection). No other drug-related clinical signs were observed.
- STORAGE CONDITIONS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
ZUPREVO™18%
(Tildipirosin)
180 mg/mLInjectable Solution for Cattle
Antimicrobial DrugFOR SUBCUTANEOUS INJECTION IN BEEF AND
NON-LACTATING DAIRY CATTLE ONLY.
NOT FOR USE IN FEMALE DAIRY CATTLE
20 MONTHS OF AGE OR OLDER OR IN CALVES
TO BE PROCESSED FOR VEAL.- 50 mL Multiple-Dose Vial
- Sterile
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.NADA 141-334, Approved by FDA
MERCK
Animal Health
-
INGREDIENTS AND APPEARANCE
ZUPREVO
tildipirosin injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-4321 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tildipirosin (UNII: S795AT66JB) (Tildipirosin - UNII:S795AT66JB) Tildipirosin 180 mg in 1 mL Inactive Ingredients Ingredient Name Strength Citric acid monohydrate (UNII: 2968PHW8QP) Propylene glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-4321-01 50 mL in 1 VIAL, MULTI-DOSE 2 NDC: 0061-4321-02 100 mL in 1 VIAL, MULTI-DOSE 3 NDC: 0061-4321-03 250 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141334 05/01/2012 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Intervet International GMBH 328855635 MANUFACTURE Establishment Name Address ID/FEI Business Operations Lonza AG 480007517 API MANUFACTURE
Trademark Results [Zuprevo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZUPREVO 77785807 4194944 Live/Registered |
Intervet Inc. 2009-07-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.