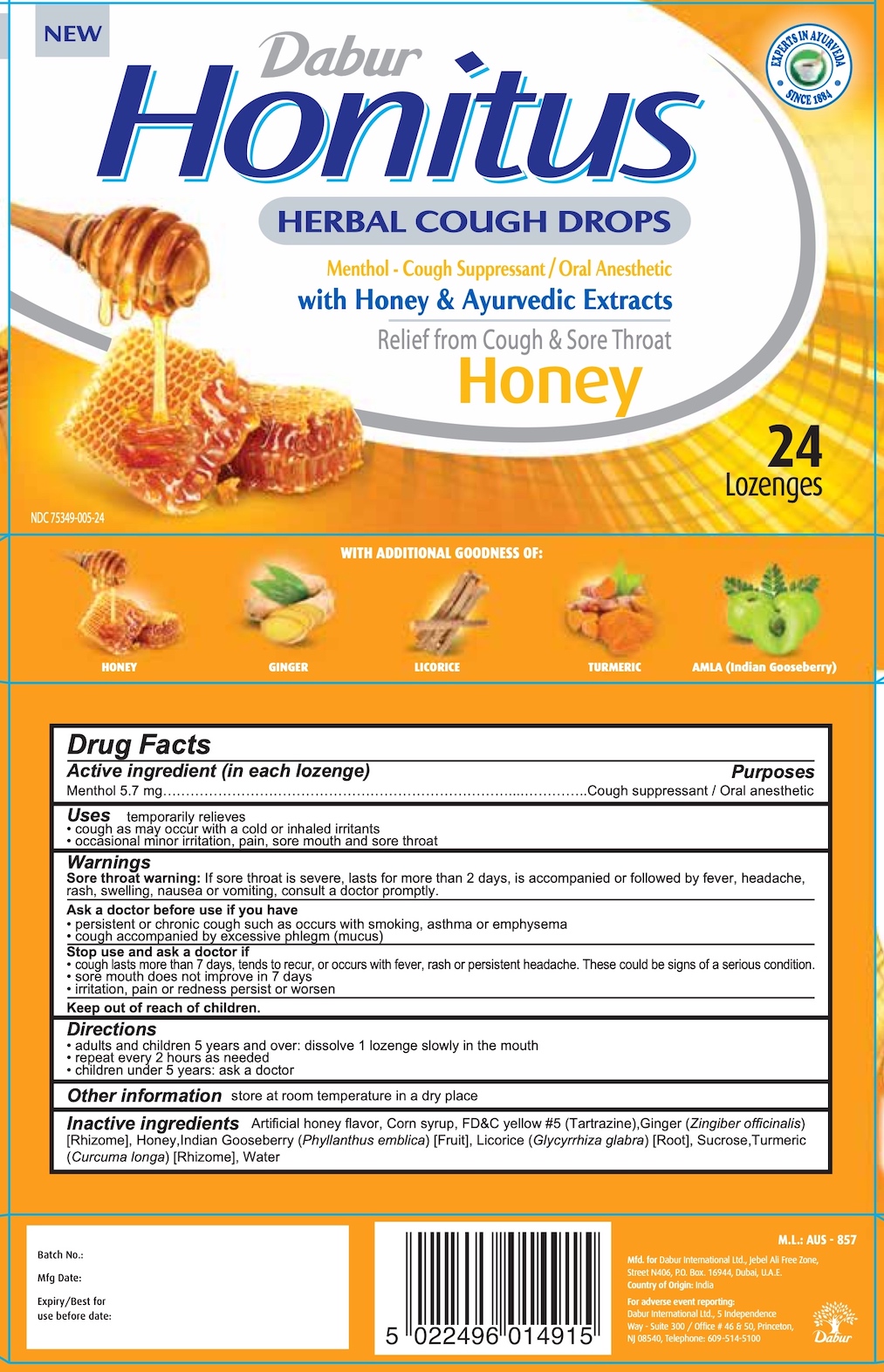
Dabur Honitus Herbal Cough Drops - Honey
Dabur Honitus Herbal Cough Drops by
Drug Labeling and Warnings
Dabur Honitus Herbal Cough Drops by is a Otc medication manufactured, distributed, or labeled by Dabur International Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DABUR HONITUS HERBAL COUGH DROPS HONEY- menthol lozenge
Dabur International Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dabur Honitus Herbal Cough Drops - Honey
Uses temporarily relieves
- cough as may occur with a cold or inhaled irritants
- occasional minor irritation, pain, sore mouth and sore throat
Warnings
Sore throat warning:
If sore throat is severe, lasts for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea or vomiting, consult a doctor promptly.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
- cough accompanied by excessive phlegm (mucus)
Directions
- adults and children 5 years and over: dissolve 1 lozenge slowly in the mouth
- repeat every 2 hours as needed
- children under 5 years: ask a doctor
Inactive ingredients
Artificial honey flavor, Corn syrup, FD& yellow #5 (Tartrazine), Ginger ( Zingiber officinalis) [Rhizome], Honey, Indian Gooseberry ( Phyllanthus emblica) [Fruit], Licorice ( Glycyrrhiza glabra) [Root], Sucrose, Turmeric ( Curcuma longa) [Rhizome], Water
Mfd. for Dabur International Ltd, Jebel Ali Free zone,
Street No. N406, P.O. Box: 16944, Dubai, U.A.E.
Country of Origin: India
For adverse event reporting: Dabur International Ltd.
5 Independence Way - Suite 300 / Office # 46 & 50
Princeton, NJ 08540, Telephone: 609-514-5100
Dabur Honitus
HERBAL COUGH DROPS
Menthol - Cough Suppressant / Oral Anesthetic
with Honey & Ayurvedic Extracts
Relief from Cough and Sore Throat
Honey
24 Lozenges
NDC: 75349-005-24
| DABUR HONITUS HERBAL COUGH DROPS
HONEY
menthol lozenge |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Dabur International Limited (851038067) |