TRUMENBA- meningococcal group b vaccine injection, suspension
Trumenba by
Drug Labeling and Warnings
Trumenba by is a Other medication manufactured, distributed, or labeled by Wyeth Pharmaceutical Division of Wyeth Holdings LLC, Pfizer Ireland Pharmaceuticals, Pfizer Health AB, Pfizer Manufacturing Belgium NV. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRUMENBA safely and effectively. See full prescribing information for TRUMENBA.
TRUMENBA® (Meningococcal Group B Vaccine)
Suspension for intramuscular injection
Initial U.S. Approval: 2014INDICATIONS AND USAGE
- Trumenba is indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. Trumenba is approved for use in individuals 10 through 25 years of age. (1)
- The effectiveness of the two-dose schedule of Trumenba against diverse N. meningitidis serogroup B strains has not been confirmed. (1)
DOSAGE AND ADMINISTRATION
- For intramuscular use only. (2)
- Three-dose schedule: Administer a dose (0.5 mL) at 0, 1–2, and 6 months. (2.1)
- Two-dose schedule: Administer a dose (0.5 mL) at 0 and 6 months. If the second dose is administered earlier than 6 months after the first dose, a third dose should be administered at least 4 months after the second dose. (2.1)
DOSAGE FORMS AND STRENGTHS
- Suspension for intramuscular injection in 0.5 mL single-dose prefilled syringe. (3)
CONTRAINDICATIONS
- Severe allergic reaction after a previous dose of Trumenba. (4)
WARNINGS AND PRECAUTIONS
Syncope (fainting) can occur in association with administration of injectable vaccines, including Trumenba. Procedures should be in place to avoid injury from fainting. (5.4)
ADVERSE REACTIONS
The most common solicited adverse reactions in adolescents and young adults were pain at the injection site (≥85%), fatigue (≥60%), headache (≥55%), and muscle pain (≥35%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Safety and effectiveness have not been established in children <10 years of age. In a clinical study, 90% of infants <12 months of age who were vaccinated with a reduced dosage formulation had fever. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Administration
2.3 Use of Trumenba with other Meningococcal Group B Vaccines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
5.2 Altered Immunocompetence
5.3 Limitation of Vaccine Effectiveness
5.4 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
14.1 Immunogenicity
14.2 Concomitant Vaccine Administration
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Trumenba is indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. Trumenba is approved for use in individuals 10 through 25 years of age.
The effectiveness of the two-dose schedule of Trumenba against diverse N. meningitidis serogroup B strains has not been confirmed.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.1 Dose and Schedule
Three-dose schedule: Administer a dose (0.5 mL) at 0, 1–2, and 6 months.
Two-dose schedule: Administer a dose (0.5 mL) at 0 and 6 months. If the second dose is administered earlier than 6 months after the first dose, a third dose should be administered at least 4 months after the second dose.
The choice of dosing schedule may depend on the risk of exposure and the patient's susceptibility to meningococcal serogroup B disease.
2.2 Administration
Shake syringe vigorously to ensure that a homogenous white suspension of Trumenba is obtained. Do not use the vaccine if it cannot be re-suspended. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is found.
Inject each 0.5 mL dose intramuscularly, using a sterile needle attached to the supplied prefilled syringe. The preferred site for injection is the deltoid muscle of the upper arm. Do not mix Trumenba with any other vaccine in the same syringe.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
Epinephrine and other appropriate agents used to manage immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur following administration of Trumenba.
5.2 Altered Immunocompetence
Reduced Immune Response
Some individuals with altered immunocompetence may have reduced immune responses to Trumenba.
Complement Deficiency
Persons with certain complement deficiencies and persons receiving treatment that inhibits terminal complement activation (for example, eculizumab) are at increased risk for invasive disease caused by N. meningitidis serogroup B even if they develop antibodies following vaccination with Trumenba [see Clinical Pharmacology (12.0)].
-
6 ADVERSE REACTIONS
In clinical studies, the most common solicited adverse reactions in adolescents and young adults were pain at the injection site (≥85%), fatigue (≥60%), headache (≥55%), and muscle pain (≥35%). Nausea was reported in up to 24% of adolescents in early phase studies.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in clinical practice.
The safety of Trumenba was evaluated in 15,227 subjects 10 through 25 years of age in 11 clinical studies (8 randomized controlled and 3 supportive non-controlled studies) conducted in the U.S., Europe, Canada, Chile, and Australia. A total of 11,333 adolescents (10 through 18 years of age) and 3,894 adults (19 through 25 years of age) received at least one dose of Trumenba. A total of 5,501 subjects 10 through 25 years of age in the control groups received saline placebo and/or one of the following vaccine(s): Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant (HPV4) (Merck & Co., Inc.); Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap) (Sanofi Pasteur Ltd.); Meningococcal Polysaccharide (Serogroups A, C, Y and W-135) Diphtheria Toxoid Conjugate Vaccine (MCV4) (Sanofi Pasteur Inc.); a non-U.S. licensed reduced diphtheria toxoid, tetanus toxoid, acellular pertussis and inactivated polio virus vaccine (dTaP-IPV) (Sanofi Pasteur, Inc.); Hepatitis A Vaccine, Inactivated (HAV) (GlaxoSmithKline Biologicals).
The safety evaluation in the clinical studies included an assessment of: (1) solicited local and systemic reactions, and use of antipyretic medication after each vaccination in an electronic diary maintained by the subject or the subject's parent/legal guardian and (2) spontaneous reports of adverse events (AEs), including serious adverse events (SAEs), throughout the study (day of vaccination through one month or 6 months after the last vaccination, depending on the study and safety parameter).
In controlled studies, demographic characteristics were generally similar with regard to gender, race, and ethnicity among subjects who received Trumenba and those who received control. Overall, across the 11 studies, among the subjects who received Trumenba, 50.5% were male and 49.5% were female, and the majority were White (86.3%) and non-Hispanic/non-Latino (87.3%).
Solicited Local and Systemic Adverse Reactions
Study 1 was a Phase 3, randomized, active-controlled, observer-blinded, multicenter trial in the U.S., Canada, and Europe in which 2,693 subjects 10 to 18 years of age received at least 1 dose of Trumenba on a 0-, 2-, and 6- month schedule. A control group (n=897) received HAV at 0 and 6 months and saline at 2 months. 87.3% of subjects were White, 8.1% were Black or African-American, 0.4% were Asian, and 5.8% were Hispanic or Latino. Overall, 51.5% of subjects were male, 55.6% of participants were 10 to 14 years age, and 44.4% were 15 to 18 years of age.
Study 2 was a Phase 3, randomized, placebo-controlled, observer-blinded, multicenter trial in the U.S., Canada, and Europe in which 2,471 subjects 18 to 25 years of age received at least 1 dose of Trumenba and 822 subjects received saline on a 0-, 2,- and 6- month schedule. 76.1% of subjects were White, 20.8% were Black or African-American, 1.6% were Asian, and 17.1% were Hispanic or Latino. Overall, 41.3% of subjects were male.
Local adverse reactions at the Trumenba injection site and control (HAV/saline or saline) injection site were assessed in both studies.
Tables 1 and 2 present the percentage and severity of reported local adverse reactions within 7 days following each dose of Trumenba or control (HAV/saline or saline) for Study 1 and Study 2, respectively.
Local adverse reactions were reported more frequently following Trumenba compared to control (see Tables 1 and 2).
Table 1: Percentages of Subjects 10 to 18 Years of Age (Study 1*) Reporting Local Adverse Reactions Within 7 Days After Each Vaccination Dose 1 Dose 2 Dose 3 Trumenba† HAV/Saline† Trumenba† HAV/Saline† Trumenba† HAV/Saline† Local Reaction N=2681 N=890 N=2545 N=843 N=2421 N=821 - * Study 1: National Clinical Trial (NCT) number NCT01830855.
- † Trumenba was administered at 0, 2, and 6 months. HAV was administered at 0 and 6 months and saline was administered at 2 months.
- ‡ Mild (does not interfere with activity); moderate (interferes with activity); severe (prevents daily activity).
- § "Any" is defined as the cumulative frequency of subjects who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- ¶ Mild (2.5–5.0 cm); moderate (>5.0–10.0 cm); severe (>10.0 cm).
Pain‡ Any§ 86.7 47.0 77.7 15.2 76.0 34.0 Mild 41.1 36.5 39.4 12.3 34.1 23.8 Moderate 40.7 9.9 33.2 2.7 36.5 9.9 Severe 5.0 0.6 5.1 0.1 5.4 0.4 Redness¶ Any§ 16.2 1.3 12.5 0.6 13.9 1.1 Mild 5.6 1.2 5.2 0.6 4.9 1.0 Moderate 8.8 0.1 6.1 0.0 6.8 0.1 Severe 1.9 0.0 1.1 0.0 2.2 0.0 Swelling¶ Any§ 18.0 2.2 13.9 0.6 15.4 0.9 Mild 8.5 1.8 6.3 0.5 7.9 0.7 Moderate 8.8 0.4 7.3 0.1 6.8 0.1 Severe 0.7 0.0 0.2 0.0 0.7 0.0 Table 2: Percentages of Subjects 18 to 25 Years of Age (Study 2*) Reporting Local Adverse Reactions Within 7 Days After Each Vaccination Dose 1 Dose 2 Dose 3 Trumenba† Saline† Trumenba† Saline† Trumenba† Saline† Local Reaction N=2425 N=798 N=2076 N=706 N=1823 N=624 - * Study 2: National Clinical Trial (NCT) number NCT01352845.
- † Trumenba was administered at 0, 2, and 6 months. Saline was administered at 0, 2, and 6 months.
- ‡ Mild (does not interfere with activity); moderate (interferes with activity); severe (prevents daily activity).
- § "Any" is defined as the cumulative frequency of subjects who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- ¶ Mild (2.5–5.0 cm); moderate (>5.0–10.0 cm); severe (>10.0 cm).
Pain‡ Any§ 84.2 11.8 79.3 7.8 80.4 6.7 Mild 42.3 10.7 42.2 6.8 36.1 6.4 Moderate 37.1 1.1 32.7 1.0 38.9 0.3 Severe 4.8 0.0 4.4 0.0 5.3 0.0 Redness¶ Any§ 13.8 0.6 11.8 0.3 17.1 0.2 Mild 5.8 0.5 4.6 0.1 6.2 0.2 Moderate 7.1 0.0 6.3 0.0 8.6 0.0 Severe 0.9 0.1 0.9 0.1 2.3 0.0 Swelling¶ Any§ 15.5 0.6 14.0 0.4 16.6 0.3 Mild 8.5 0.3 7.7 0.3 8.8 0.0 Moderate 6.8 0.3 6.0 0.1 7.2 0.3 Severe 0.2 0.1 0.3 0.0 0.5 0.0 In Study 1, mean duration of pain was 2.4 to 2.6 days (range 1–17 days), for redness 2.0 to 2.2 days (range 1–12 days) and for swelling 2.0 to 2.1 days (range 1–21 days) in the combined Trumenba group. In Study 2, mean duration of pain was 2.6 to 2.8 days (range 1–67 days), for redness 2.2 to 2.5 days (range 1–13 days) and for swelling 2.1 to 2.6 days (range 1–70 days) in the Trumenba group.
Tables 3 and 4 present the percentage and severity of reported solicited systemic adverse reactions within 7 days of each dose of Trumenba or control (HAV/saline or saline) for Study 1 and Study 2, respectively.
Table 3: Percentages of Subjects 10 to 18 Years of Age (Study 1*) Reporting Systemic Adverse Reactions and Use of Antipyretic Medications Within 7 Days After Each Vaccination Dose 1 Dose 2 Dose 3 Trumenba† HAV/Saline† Trumenba† HAV/Saline† Trumenba† HAV/Saline† Systemic Reaction N=2681 N=890 N=2545 N=843 N=2421 N=821 - * Study 1: National Clinical Trial (NCT) number NCT01830855.
- † Trumenba was administered at 0, 2, and 6 months. HAV was administered at 0 and 6 months and saline was administered at 2 months.
- ‡ Study 1: Fever (≥38°C): N=2679, 2540, and 2414 for Trumenba at Dose 1, Dose 2, and Dose 3, respectively; N=890, 840, and 819 for HAV/saline at Dose 1, Dose 2, and Dose 3, respectively.
- § Mild (1–2 times in 24 hours); moderate (>2 times in 24 hours); severe (requires intravenous hydration).
- ¶ "Any" is defined as the cumulative frequency of subjects who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- # Mild (2–3 loose stools in 24 hours); moderate (4–5 loose stools in 24 hours); severe (6 or more loose stools in 24 hours).
- Þ Mild (does not interfere with activity); moderate (interferes with activity); severe (prevents daily activity).
Fever (≥38°C)‡ ≥38.0°C 6.4 1.9 2.0 1.5 2.7 2.3 38.0°C to <38.5°C 4.0 1.3 1.2 0.7 1.8 1.3 38.5°C to <39.0°C 1.9 0.3 0.7 0.7 0.6 0.4 39.0°C to ≤40.0°C 0.5 0.2 0.1 0.1 0.3 0.5 >40.0°C 0.0 0.0 0.0 0.0 0.0 0.1 Vomiting§ Any¶ 3.7 1.9 2.2 1.4 1.7 2.2 Mild 2.8 1.7 1.7 1.1 1.4 1.7 Moderate 0.9 0.2 0.4 0.4 0.3 0.5 Severe 0.0 0.0 0.0 0.0 0.0 0.0 Diarrhea# Any¶ 10.6 12.1 7.6 9.1 7.7 7.6 Mild 9.1 10.9 6.2 7.6 6.4 6.2 Moderate 1.3 1.1 1.3 1.2 1.0 1.1 Severe 0.3 0.1 0.1 0.4 0.3 0.2 HeadacheÞ Any¶ 51.8 37.2 37.8 28.1 35.4 24.8 Mild 28.7 24.0 20.2 15.7 18.9 13.5 Moderate 21.0 12.5 16.0 10.9 15.2 10.4 Severe 2.2 0.7 1.7 1.5 1.3 1.0 FatigueÞ Any¶ 54.0 40.3 38.3 26.3 35.9 24.4 Mild 27.8 23.5 20.6 13.2 18.4 13.5 Moderate 23.2 15.2 15.8 11.7 15.2 10.0 Severe 3.0 1.7 1.9 1.4 2.3 0.9 ChillsÞ Any¶ 25.3 17.2 16.0 10.3 13.1 8.3 Mild 16.2 13.3 10.6 8.1 8.7 6.5 Moderate 8.0 3.5 4.8 1.8 3.8 1.7 Severe 1.2 0.4 0.6 0.5 0.5 0.1 Muscle pain (other than muscle pain at the injection site)Þ Any¶ 24.4 19.2 17.8 10.3 17.6 11.1 Mild 13.2 13.5 8.7 5.2 9.5 6.6 Moderate 10.1 5.4 7.9 4.5 7.2 4.3 Severe 1.2 0.3 1.2 0.6 0.8 0.2 Joint painÞ Any¶ 21.9 13.6 16.7 9.1 16.0 8.9 Mild 11.8 8.3 8.4 5.0 8.9 5.5 Moderate 8.7 4.6 7.5 3.4 5.9 3.0 Severe 1.4 0.7 0.8 0.7 1.2 0.4 Use of antipyretic medication 20.7 10.4 13.6 8.9 12.7 6.8 Table 4: Percentages of Subjects 18 to 25 Years of Age (Study 2*) Reporting Systemic Adverse Reactions and Use of Antipyretic Medications Within 7 Days After Each Vaccination Dose 1 Dose 2 Dose 3 Trumenba† Saline† Trumenba† Saline† Trumenba† Saline† Systemic Reaction N=2425 N=798 N=2076 N=706 N=1823 N=624 - * Study 2: National Clinical Trial (NCT) number NCT01352845.
- † Trumenba was administered at 0, 2, and 6 months. Saline was administered at 0, 2, and 6 months.
- ‡ Study 2: Fever (≥38°C): N=2415, 2067, and 1814 for Trumenba at Dose 1, Dose 2, and Dose 3, respectively; N=796, 705, and 621 for saline at Dose 1, Dose 2, and Dose 3, respectively.
- § Mild (1–2 times in 24 hours); moderate (>2 times in 24 hours); severe (requires intravenous hydration).
- ¶ "Any" is defined as the cumulative frequency of subjects who reported a reaction as "mild", "moderate", or "severe" within 7 days of vaccination.
- # Mild (2–3 loose stools in 24 hours); moderate (4–5 loose stools in 24 hours); severe (6 or more loose stools in 24 hours).
- Þ Mild (does not interfere with activity); moderate (interferes with activity); severe (prevents daily activity).
Fever (≥38°C)‡ ≥38.0°C 2.4 0.6 1.2 1.0 2.0 0.6 38.0°C to <38.5°C 1.6 0.4 0.7 0.6 1.4 0.5 38.5°C to <39.0°C 0.7 0.0 0.4 0.3 0.4 0.2 39.0°C to ≤40.0°C 0.0 0.3 0.1 0.1 0.1 0.0 >40.0°C 0.0 0.0 0.0 0.0 0.1 0.0 Vomiting§ Any¶ 2.6 2.1 2.1 1.6 2.0 1.4 Mild 2.2 2.1 1.6 1.3 1.8 1.1 Moderate 0.4 0.0 0.5 0.3 0.2 0.3 Severe 0.0 0.0 0.0 0.0 0.0 0.0 Diarrhea# Any¶ 12.7 11.8 8.6 8.1 7.5 6.9 Mild 10.2 9.8 6.4 4.7 6.1 5.3 Moderate 2.4 1.9 1.7 2.8 1.2 1.3 Severe 0.2 0.1 0.5 0.6 0.2 0.3 HeadacheÞ Any¶ 43.9 36.2 33.1 24.9 32.5 21.6 Mild 24.3 22.1 18.4 13.6 17.6 12.5 Moderate 17.9 13.5 13.3 10.1 13.3 8.3 Severe 1.6 0.6 1.4 1.3 1.6 0.8 FatigueÞ Any¶ 50.9 39.8 39.2 27.3 39.3 24.5 Mild 25.4 23.2 20.6 13.9 18.9 13.1 Moderate 22.1 15.8 16.4 11.5 18.8 9.6 Severe 3.4 0.9 2.2 2.0 1.6 1.8 ChillsÞ Any¶ 18.1 9.8 12.4 8.5 12.6 6.4 Mild 12.0 8.1 8.1 6.9 7.7 4.3 Moderate 4.9 1.6 3.5 1.6 4.2 2.1 Severe 1.1 0.0 0.8 0.0 0.8 0.0 Muscle pain (other than muscle pain at the injection site)Þ Any¶ 25.9 14.5 15.6 8.5 16.9 7.5 Mild 13.0 9.6 7.6 5.8 8.9 4.5 Moderate 11.3 4.4 7.1 2.3 6.8 2.9 Severe 1.6 0.5 0.8 0.4 1.2 0.2 Joint painÞ Any¶ 19.6 10.9 15.1 6.5 12.6 5.3 Mild 10.3 6.9 8.1 3.7 6.6 2.9 Moderate 7.9 3.5 6.2 2.5 5.4 2.4 Severe 1.4 0.5 0.9 0.3 0.6 0.0 Use of antipyretic medication 13.4 8.9 12.3 7.6 12.8 6.6 The frequencies of adverse reactions were highest after the first dose regardless of the schedule. After subsequent doses, the frequencies of adverse reactions were similar regardless of dose number and schedule.
Serious Adverse Events
Overall in clinical studies in which 15,227 subjects 10 through 25 years of age received at least one dose of Trumenba, serious adverse events (SAEs) were reported by 269 (1.8%) subjects.
Among the 8 controlled studies (Trumenba N=13,275, control N=5,501), SAEs were reported by 213 (1.6%) subjects and by 106 (1.9%) subjects who received at least one dose of Trumenba or control, respectively.
Non-serious Adverse Events
Overall in clinical studies in which 15,227 subjects 10 through 25 years of age received Trumenba, non-serious AEs within 30 days after any dose were reported in 4,463 (29.3%) subjects. Among the 8 controlled studies (Trumenba N=13,275, control N=5,501), AEs that occurred within 30 days of vaccination were reported in 4,056 (30.6%) subjects who received Trumenba and 1,539 (28.0%) subjects in the control group, for individuals who received at least one dose. AEs that occurred at a frequency of at least 2% and were more frequently observed in subjects who received Trumenba than subjects in the control group were injection site pain, fever, and headache.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Trumenba. Because these reactions are reported voluntarily froma population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Immune System Disorders: Hypersensitivity reactions, including anaphylactic reactions.
Nervous system disorder: Syncope (fainting).
-
7 DRUG INTERACTIONS
In clinical trials, Trumenba was administered concomitantly with HPV4 in adolescents 11 to <18 years of age and with MCV4 and Tdap in adolescents 10 to <13 years of age [see Clinical Studies (14.0) and Adverse Reactions (6.0)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. There are no adequate and well-controlled studies of Trumenba in pregnant women. Available human data on Trumenba administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
Two developmental toxicity studies were performed in female rabbits administered Trumenba prior to mating and during gestation. The dose was 0.5 mL at each occasion (a single human dose is 0.5 mL). These studies revealed no evidence of harm to the fetus or offspring (until weaning) due to Trumenba [see Animal Data].
Animal Data
Two developmental toxicity studies were performed in female rabbits. Animals were administered Trumenba by intramuscular injection 17 days and 4 days prior to mating and on gestation Days 10 and 24. The dose was 0.5 mL at each occasion (a single human dose is 0.5 mL). No adverse effects on pre-weaning development up to post-natal day 21 were observed. There were no fetal malformations or variations observed due to the vaccine.
8.2 Lactation
Risk Summary
Available data are not sufficient to assess the effects of Trumenba on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Trumenba and any potential adverse effects on the breastfed child from Trumenba or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
-
11 DESCRIPTION
Trumenba is a sterile suspension composed of two recombinant lipidated factor H binding protein (fHBP) variants from N. meningitidis serogroup B, one from fHBP subfamily A and one from subfamily B (A05 and B01, respectively).1 The proteins are individually produced in E. coli. Production strains are grown in defined fermentation growth media to a specific density. The recombinant proteins are extracted from the production strains and purified through a series of column chromatography steps. Polysorbate 80 (PS80) is added to the drug substances and is present in the final drug product.
Each 0.5 mL dose contains 60 micrograms of each fHBP variant (total of 120 micrograms of protein), 0.018 mg of PS80 and 0.25 mg of Al3+ as AlPO4 in 10 mM histidine buffered saline at pH 6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Protection against invasive meningococcal disease is conferred mainly by complement-mediated antibody-dependent killing of N. meningitidis. The effectiveness of Trumenba was assessed by measuring serum bactericidal activity using human complement (hSBA).
fHBP is one of many proteins found on the surface of meningococci and contributes to the ability of the bacterium to avoid host defenses. fHBPs can be categorized into two immunologically distinct subfamilies, A and B.1 The susceptibility of serogroup B meningococci to complement-mediated antibody-dependent killing following vaccination with Trumenba is dependent on both the antigenic similarity of the bacterial and vaccine fHBPs, as well as the amount of fHBP expressed on the surface of the invading meningococci.
-
13 NONCLINICAL TOXICOLOGY
Trumenba has not been evaluated for carcinogenic or mutagenic potential or impairment of fertility in males. Vaccination of female rabbits with Trumenba had no effect on fertility [see Pregnancy (8.1)].
-
14 CLINICAL STUDIES
The immunogenicity of Trumenba following the three-dose schedule (0, 2, and 6 months) was evaluated in individuals 10 to 25 years of age in the U.S., Canada, and Europe (Studies 1 and 2) and following the two-dose (0 and 6 months) and three-dose schedules (0, 1–2, and 6 months) in individuals 11 to 18 years of age in Europe (Study 3). Serum bactericidal antibodies were measured with hSBA assays that used each of four meningococcal serogroup B strains. These four primary test strains express fHBP variants representing the two subfamilies (A and B) and, when taken together, are representative of meningococcal serogroup B strains causing invasive disease in the U.S. and Europe. The studies assessed the proportions of subjects with a 4-fold or greater increase in hSBA titer for each of the four primary strains. The studies also assessed the composite response to the four primary strains combined (proportion of subjects who achieved a hSBA titer greater than or equal to 1:8 (three strains) or 1:16 (one strain). To assess the effectiveness of the three-dose schedule of Trumenba against diverse meningococcal serogroup B strains, the proportion of subjects achieving a defined hSBA titer post-dose 3 was evaluated against a panel of 10 additional strains, each expressing a different fHBP variant.
14.1 Immunogenicity
The hSBA responses to each of the primary strains observed in U.S. subjects after the third dose of Trumenba are presented for Study 1 and Study 2 in Table 5.
Table 5: Percentages of U.S. Individuals 10 to 25 Years of Age With ≥4-fold rise in hSBA Titer and Composite Response Following Administration of Trumenba on a 0-, 2-, and 6-Month Schedule for Four Primary Strains (Studies 1 and 2)*,†,‡,§ Study 1 Study 2 (10 to 18 Years of Age) (18 to 25 Years of Age) n %
(95% CI)¶n %
(95% CI)¶Subfamily/Subgroup
fHBP Variant#,ÞAbbreviations: CI=confidence interval; fHBP=factor H binding protein; hSBA=serum bactericidal assay using human complement; LLOQ=lower limit of quantitation; LOD=limit of detection. Note: LLOQ = 1:16 for A22; 1:8 for A56, B24, and B44. Note: The 4-fold increase is defined as follows: (1) For subjects with a baseline hSBA titer <1:4, a response is defined as an hSBA titer ≥1:16. (2) For subjects with a baseline hSBA titer ≥1:4, a response is defined as an hSBA titer ≥4 times the LLOQ or ≥4 times the baseline titer, whichever was higher. Note: Pre-specified criteria for assessment of hSBA responses (4-fold rise in titer to each primary test strain, and titer above LLOQ for all four primary test strains) among U.S. subjects were met in these studies. - * Evaluable immunogenicity population.
- † Study 1 = NCT01830855 and Study 2 = NCT01352845.
- ‡ Study 1: Group 1 (0, 2, and 6 months).
- § Study 2: Group 1 (0, 2, and 6 months).
- ¶ Exact 2-sided confidence interval (Clopper-Pearson method) based upon the observed proportion of subjects.
- # The strains expressing variants A22, A56, B24, and B44 correspond to strains PMB80, PMB2001, PMB2948, and PMB2707, respectively.
- Þ For the third dose, serum was obtained approximately 1 month after vaccination.
- ß Composite response = hSBA ≥ LLOQ for all 4 primary meningococcal B strains.
≥4-Fold Increase PMB80 (A22) Dose 3 587 86.2
(83.1, 88.9)644 81.1
(77.8, 84.0)PMB2001 (A56) Dose 3 526 92.0
(89.4, 94.2)621 90.7
(88.1, 92.8)PMB2948 (B24) Dose 3 585 81.9
(78.5, 84.9)634 83.9
(80.8, 86.7)PMB2707 (B44) Dose 3 555 88.3
(85.3, 90.8)643 79.3
(76.0, 82.4)Composite hSBA responseß Before Dose 1 507 0.6
(0.1, 1.7)610 3.3
(2.0, 5.0)Dose 3 537 85.7
(82.4, 88.5)625 82.4
(79.2, 85.3)The hSBA responses against a panel of 10 additional strains observed in U.S. subjects after the third dose of Trumenba are presented for Study 1 and Study 2 in Table 6.
Table 6. Percentages of U.S. Individuals 10 to 25 Years of Age With a hSBA Titer ≥ LLOQ Against 10 Additional Strains Following Administration of Trumenba on a 0-, 2-, and 6-Month Schedule (Study 1 and Study 2)- *,† Study 1 Study 2 (10 to 18 Years of Age) (18 to 25 Years of Age) n %
(95% CI)‡n %
(95% CI)‡Subfamily/Subgroup
fHBP Variant§,¶Abbreviations: CI=confidence interval; fHBP=factor H binding protein; hSBA=serum bactericidal assay using human complement; LLOQ=lower limit of quantitation. Note: LLOQ = 1:16 for A06, A12, and A19; 1:8 for A07, A15, A29, B03, B09, B15, and B16. - * The evaluable immunogenicity population was used for the analysis.
- † Study 1 = NCT01830855 and Study 2 = NCT01352845.
- ‡ Exact 2-sided confidence interval (Clopper and Pearson) based upon the observed proportion of subjects.
- § The strains expressing variants A06, A12, A19, A07, A15, A29, B03, B09, B15, and B16 correspond to strains PMB3010, PMB824, PMB1989, PMB3040, PMB1672, PMB3175, PMB1256, PMB866, PMB431, and PMB648, respectively.
- ¶ For the third dose, serum was obtained approximately 1 month after vaccination.
A/N1C1
PMB3175 (A29)Before Dose 1 169 11.2
(6.9, 17.0)160 23.8
(17.4, 31.1)Dose 3 176 98.9
(96.0, 99.9)162 98.8
(95.6, 99.9)A/N1C2
PMB3010 (A06)Before Dose 1 178 7.9
(4.4, 12.8)166 10.8
(6.6, 16.6)Dose 3 179 97.8
(94.4, 99.4)164 89.0
(83.2, 93.4)A/N2C1
PMB3040 (A07)Before Dose 1 170 37.6
(30.3, 45.4)165 55.8
(47.8, 63.5)Dose 3 178 96.1
(92.1, 98.4)165 95.2
(90.7, 97.9)PMB824 (A12) Before Dose 1 180 5.0
(2.3, 9.3)166 4.8
(2.1, 9.3)Dose 3 180 76.1
(69.2, 82.1)165 66.7
(58.9, 73.8)PMB1672 (A15) Before Dose 1 170 15.9
(10.7, 22.3)159 30.2
(23.2, 38.0)Dose 3 166 86.7
(80.6, 91.5)159 89.9
(84.2, 94.1)A/N2C2
PMB1989 (A19)Before Dose 1 174 5.7
(2.8, 10.3)158 23.4
(17.1, 30.8)Dose 3 173 91.9
(86.8, 95.5)163 94.5
(89.8, 97.4)B/N6
PMB1256 (B03)Before Dose 1 183 2.2
(0.6, 5.5)164 5.5
(2.5, 10.2)Dose 3 181 92.3
(87.4, 95.7)161 84.5
(77.9, 89.7)PMB866 (B09) Before Dose 1 180 12.2
(7.8, 17.9)165 13.9
(9.0, 20.2)Dose 3 182 85.7
(79.8, 90.5)162 72.2
(64.7, 79.0)PMB431 (B15) Before Dose 1 180 27.8
(21.4, 34.9)163 33.1
(26.0, 40.9)Dose 3 183 97.3
(93.7, 99.1)163 95.7
(91.4, 98.3)PMB648 (B16) Before Dose 1 180 6.7
(3.5, 11.4)161 11.8
(7.3, 17.8)Dose 3 180 83.9
(77.7, 88.9)159 72.3
(64.7, 79.1)In Study 3, Trumenba was administered according to different schedules, including Group 1 (0, 1, and 6 months), Group 2 (0, 2, and 6 months) and Group 3 (0 and 6 months). The hSBA responses observed after the second dose in Groups 1, 2, and 3 and completion of the three-dose series in Group 1 and 2 are presented in Table 7.
Table 7: Percentages of European Individuals 11 to 18 Years of Age With a ≥4-Fold Increase in hSBA Titer and Composite Response*,† Group 1 Group 2 Group 3 3-Dose Schedule
(0, 1, and 6 Months)‡3-Dose Schedule
(0, 2, and 6 Months)§2-Dose Schedule
(0 and 6 Months)¶fHBP Variant#,Þ %
(95% CI)ß%
(95% CI)ß%
(95% CI)ßAbbreviations: CI=confidence interval; fHBP=factor H binding protein; hSBA=serum bactericidal assay using human complement; LLOQ=lower limit of quantitation; NA=not applicable. Note: LLOQ = 1:16 for PMB80 (A22) and 1:8 for PMB2001 (A56), PMB2948 (B24), and PMB2707 (B44). Note: The ≥4-fold increase is defined as follows: (1) For subjects with a baseline hSBA titer <1:4, a ≥4-fold increase was defined as an hSBA titer ≥1:16. (2) For subjects with a baseline hSBA titer ≥1:4, a ≥4-fold increase was defined as an hSBA titer ≥4 times the LLOQ or ≥4 times the baseline titer, whichever was higher. - * Per-schedule Evaluable populations. Dose 2 data include subjects who received two doses, irrespective of whether they received the third dose.
- † Study 3: NCT01299480.
- ‡ Group 1 (0, 1, and 6 months). The denominators ranged from 173 to 187 after Dose 2 and 178 to 188 after Dose 3, depending on the strain.
- § Group 2 (0, 2, and 6 months). The denominators ranged from 229 to 240 after Dose 2 and 159 to 162 after Dose 3, depending on the strain.
- ¶ Group 3 (0 and 6 months). The denominators ranged from 188 to 203 after Dose 2, depending on the strain.
- # The strains expressing variant A22, A56, B24, and B44 correspond to strains PMB80, PMB2001, PMB2948, and PMB2707, respectively.
- Þ For the second and third doses, serum was obtained approximately 1 month after vaccination.
- ß Exact 2-sided confidence interval (Clopper and Pearson) based upon the observed proportion of subjects.
- à Composite response = hSBA ≥LLOQ for all 4 primary meningococcal B strains.
≥4-Fold Increase PMB80 (A22) Dose 2 58.8
(51.4, 66.0)72.5
(66.4, 78.0)82.3
(76.3, 87.3)Dose 3 77.6
(70.9, 83.4)87.7
(81.6, 92.3)NA PMB2001 (A56) Dose 2 87.8
(82.2, 92.2)90.7
(86.2, 94.1)90.1
(85.1, 93.8)Dose 3 91.2
(86.1, 94.9)93.8
(88.8, 97.0)NA PMB2948 (B24) Dose 2 51.1
(43.6, 58.5)54.2
(47.7, 60.7)64.5
(57.4, 71.1)Dose 3 74.1
(67.1, 80.2)78.3
(71.1, 84.4)NA PMB2707 (B44) Dose 2 48.1
(40.7, 55.6)53.4
(46.8, 59.9)66.0
(58.9, 72.6)Dose 3 80.9
(74.5, 86.2)78.6
(71.4, 84.7)NA Composite ResponseÞ,à Before Dose 1 4.6
(2.0, 8.8)2.2
(0.7, 5.0)1.5
(0.3, 4.4)Dose 2 52.0
(44.3, 59.7)52.0
(45.3, 58.6)72.9
(65.9, 79.1)Dose 3 80.3
(73.7, 85.9)81.8
(74.9, 87.4)NA 14.2 Concomitant Vaccine Administration
Study 4 evaluated the immunogenicity of concomitantly administered Trumenba and Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant (HPV4) (Merck & Co, Inc.). U.S. subjects 11 to <18 years of age were randomized into three groups: Group 1 received Trumenba and HPV4 (N=992), Group 2 received Trumenba and saline (N=990), and Group 3 received saline and HPV4 (N=501). All vaccines were administered according to a 0, 2 and 6 month schedule. Immune responses were evaluated by comparisons of geometric mean titer [GMT] for each HPV type at 1 month after the third HPV4 vaccination (Group 1 vs. Group 3), and hSBA GMTs using two meningococcal serogroup B strains [variants A22 and B24] 1 month after the third Trumenba vaccination (Group 1 vs. Group 2). The noninferiority criteria for the comparisons of GMTs [lower limit of the 2-sided 95% confidence interval (CI) of the GMT ratio (Group 1/Group 3 for HPV and Group 1/Group 2 for meningococcal serogroup B strains) >0.67] were met for three HPV types (6, 11 and 16) and for the meningococcal serogroup B strains tested. For HPV-18, the lower bound of the 95% CI for the GMT ratio was 0.62 at one month after the third HPV4 vaccination.
Study 5 evaluated the immunogenicity of concomitantly administered Trumenba and Meningococcal Polysaccharide (Serogroups A, C, Y and W-135) Diphtheria Toxoid Conjugate Vaccine (MCV4) (Sanofi Pasteur Inc.) and Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap) (Sanofi Pasteur Ltd.) vaccines. U.S. subjects 10 to <13 years of age were randomized into three groups: Group 1 received Trumenba at 0, 2, and 6 months, and MCV4 and Tdap were coadministered with the first Trumenba dose (N=883). Group 2 received saline at 0, 2 and 6 months, and MCV4 and Tdap were coadministered with the first saline injection (N=870). Group 3 received Trumenba at 0, 2 and 6 months, and saline was coadministered with the first Trumenba dose (N=875). Immune responses were evaluated by comparisons of GMTs for each of the MCV4 and Tdap antigens 1 month after the first Trumenba vaccination, and hSBA GMTs using two meningococcal serogroup B strains [variants A22 and B24] 1 month after the third Trumenba vaccination. The noninferiority criteria for the comparisons of GMTs [lower limit of the 2-sided 95% CI of the GMT ratio (Group 1/Group 3 for meningococcal serogroup B strains and Group 1/Group 2 for MCV4 and Tdap) >0.67] were met for all antigens.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Trumenba is supplied in the following strengths and package configurations:
Prefilled Syringe, 1 Dose (10 per package) – NDC: 0005-0100-10.
Prefilled Syringe, 1 Dose (5 per package) – NDC: 0005-0100-05.
Prefilled Syringe, 1 Dose (1 per package) – NDC: 0005-0100-02 (This Package Not for Sale).
After shipping, Trumenba may arrive at temperatures between 2°C to 25°C (36°F to 77°F).
The tip cap and rubber plunger of the prefilled syringe are not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
Prior to administration of this vaccine, the healthcare professional should inform the individual, parent, guardian, or other responsible adult of the following:
- The importance of completing the immunization series.
- Report any suspected adverse reactions to a healthcare professional.
Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).

U.S. Govt. License No. 3
LAB-0722-9.0
CPT Code 90621 -
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
NDC: 0005-0100-01
Rx onlyMeningococcal
Group B Vaccine
Trumenba®One Dose (0.5 mL) FOR IM USE ONLY
REFRIGERATE
DO NOT FREEZE
SHAKE VIGOROUSLYWyeth Pharm. Inc
US Govt. License No. 3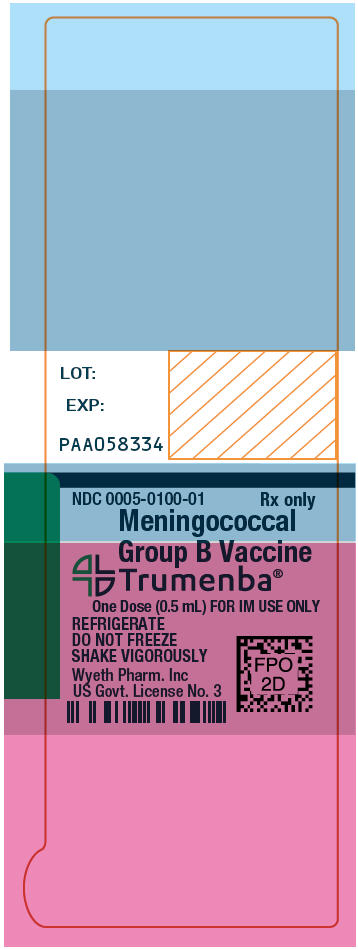
-
PRINCIPAL DISPLAY PANEL - 5 - 0.5 mL Syringe Carton
NDC: 0005-0100-05
Meningococcal
Group B VaccineTrumenba®
For use in individuals
10 through 25 years of age5 One-Dose (0.5 mL)
Prefilled SyringesPfizer
FOR INTRAMUSCULAR USE ONLY
Rx only
-
PRINCIPAL DISPLAY PANEL - 10 - 0.5 mL Syringe Carton
NDC: 0005-0100-10
Meningococcal
Group B VaccineTrumenba®
For use in individuals
10 through 25 years of age10 One-Dose (0.5 mL)
Prefilled SyringesPfizer
FOR INTRAMUSCULAR USE ONLY
Rx only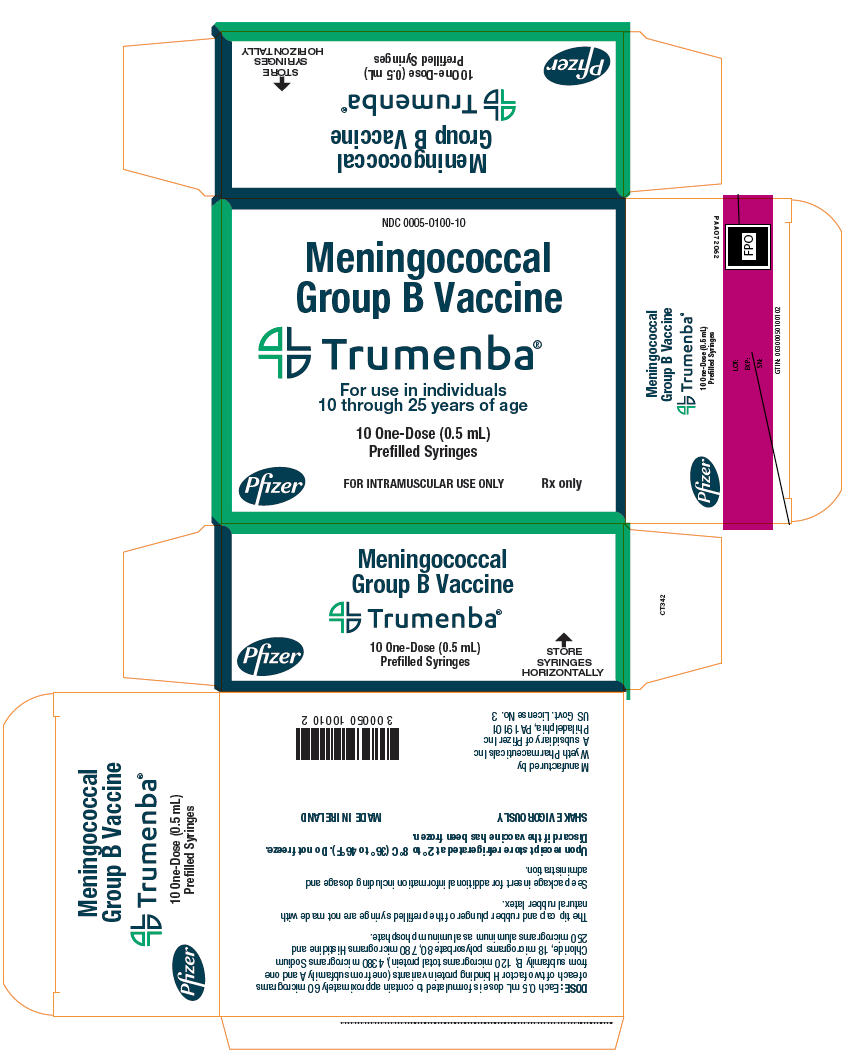
-
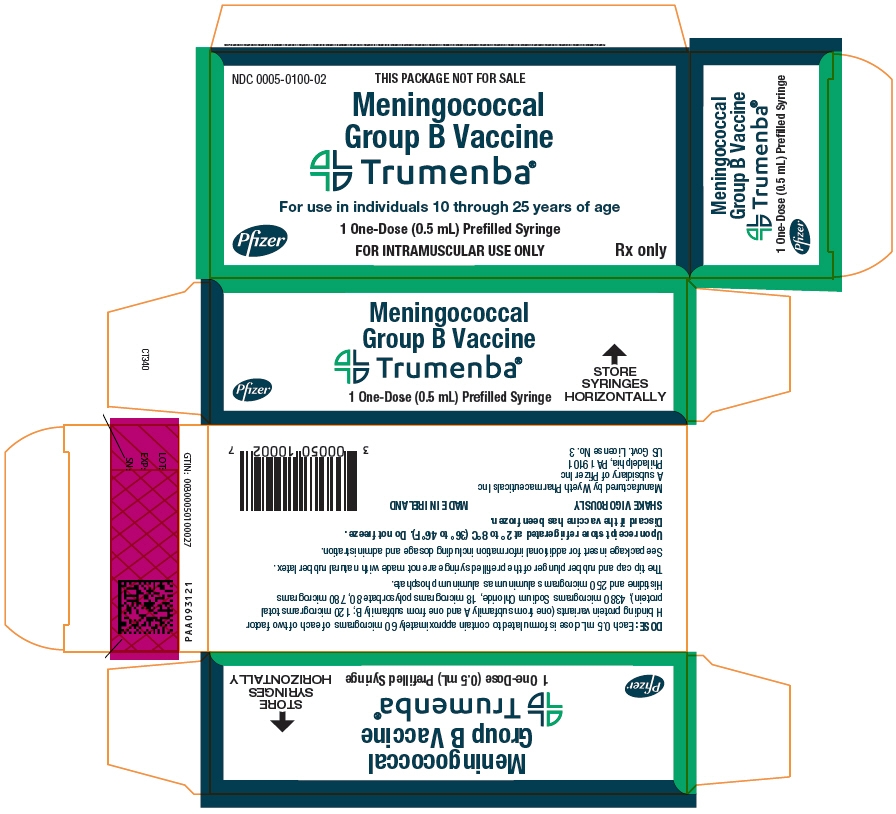
PRINCIPAL DISPLAY PANEL - 1 - 0.5 mL Syringe Carton
NDC: 0005-0100-02
THIS PACKAGE NOT FOR SALE
Meningococcal
Group B VaccineTrumenba®
For use in individuals 10 through 25 years of age
Pfizer
1 One-Dose (0.5 mL) Prefilled Syringe
FOR INTRAMUSCULAR USE ONLY
Rx only
-
INGREDIENTS AND APPEARANCE
TRUMENBA
meningococcal group b vaccine injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 0005-0100 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN (UNII: 583WCD0IZI) (NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN - UNII:583WCD0IZI) NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 A05 PROTEIN VARIANT ANTIGEN 60 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN (UNII: 7MBD4K530D) (NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN - UNII:7MBD4K530D) NEISSERIA MENINGITIDIS GROUP B RECOMBINANT LP2086 B01 PROTEIN VARIANT ANTIGEN 60 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.018 mg in 0.5 mL ALUMINUM PHOSPHATE (UNII: F92V3S521O) 0.25 mg in 0.5 mL HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0005-0100-05 5 in 1 CARTON 1 NDC: 0005-0100-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0005-0100-10 10 in 1 CARTON 2 NDC: 0005-0100-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC: 0005-0100-02 1 in 1 CARTON 3 NDC: 0005-0100-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125549 11/05/2014 Labeler - Wyeth Pharmaceutical Division of Wyeth Holdings LLC (054065909) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals 985586408 ANALYSIS(0005-0100) , MANUFACTURE(0005-0100) Establishment Name Address ID/FEI Business Operations John Wyeth & Brother Ltd. 215193306 LABEL(0005-0100) , PACK(0005-0100) Establishment Name Address ID/FEI Business Operations Wyeth Pharmaceutical Division of Wyeth Holdings LLC 054065909 ANALYSIS(0005-0100) Establishment Name Address ID/FEI Business Operations Wyeth Pharmaceutical Division of Wyeth Holdings LLC 883534067 ANALYSIS(0005-0100) , API MANUFACTURE(0005-0100)
Trademark Results [Trumenba]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRUMENBA 85035452 4384268 Live/Registered |
Wyeth LLC 2010-05-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.