K-100 antiseptic and disinfectant
K-100 by
Drug Labeling and Warnings
K-100 by is a Otc medication manufactured, distributed, or labeled by JOAQUIN ARMANDO CARDENAS URQUIDEZ. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
K-100- hydrogen peroxide solution
JOAQUIN ARMANDO CARDENAS URQUIDEZ
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
K-100 antiseptic and disinfectant
INDICATIONS AND USAGE SECTION
Clean the wound with soap and water and pat dry with sterile gauze pad.
Disinfect the wound with k-100 by applying it directly to the wound and allowing the product to dry out completely.
allow it to act for 5 minutes then wipe dry any excess product with a cloth or rag no need to rinse.
DOSAGE AND ADMINISTRATION
Clean the wound with soap and water and pat dry with sterile gauze pad.
Disinfect the wound with k-100 by applying it directly to the wound and allowing the product to dry out completely.
allow it to act for 5 minutes then wipe dry any excess product with a cloth or rag no need to rinse.
| K-100
hydrogen peroxide solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - JOAQUIN ARMANDO CARDENAS URQUIDEZ (951588416) |
| Registrant - JOAQUIN ARMANDO CARDENAS URQUIDEZ (951588416) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| JOAQUIN ARMANDO CARDENAS URQUIDEZ | 951588416 | manufacture(81577-4000) | |
Trademark Results [K-100]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
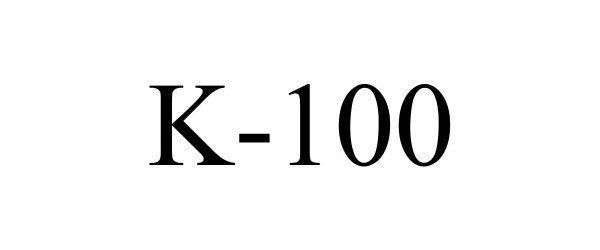 K-100 88930739 not registered Live/Pending |
Chuong Le Huynh 2020-05-23 |
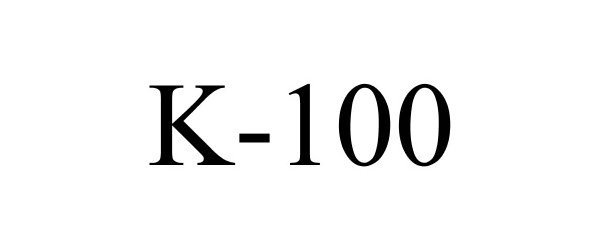 K-100 86054154 4514126 Live/Registered |
International Flora Technologies, Ltd. 2013-09-03 |
 K-100 73560986 1398236 Live/Registered |
ALPASE, INC. 1985-10-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


