DECITABINE for injection, for intravenous use
Decitabine by
Drug Labeling and Warnings
Decitabine by is a Prescription medication manufactured, distributed, or labeled by Zydus Lifesciences Limited, Zydus Pharmaceuticals USA Inc., Zydus Hospira Oncology Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DECITABINE- decitabine injection, powder, lyophilized, for solution
Zydus Lifesciences Limited
----------
DECITABINE for injection, for intravenous use
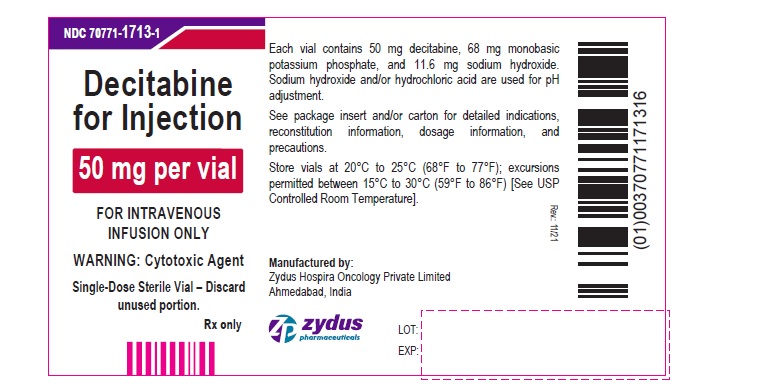
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 70771-1713-1
Decitabine for Injection
50 mg per vial
FOR INTRAVENOUS INFUSION ONLY
WARNING: Cytotoxic Agent
Single-Dose Sterile Vial – Discard unused portion.
Rx only
NDC: 70771-1713-1
Decitabine for Injection
50 mg per vial
FOR INTRAVENOUS INFUSION ONLY
WARNING: Cytotoxic Agent
Single-Dose Sterile Vial – Discard unused portion.
Rx only
| DECITABINE
decitabine injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (918596198) |
| Registrant - Zydus Pharmaceuticals USA Inc. (156861945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Hospira Oncology Private Limited | 676190889 | ANALYSIS(70771-1713) , LABEL(70771-1713) , MANUFACTURE(70771-1713) , PACK(70771-1713) | |
Revised: 5/2025
Document Id: 57e2b084-81c3-45e5-a28f-607385c0550f
Set id: bcda3a7c-f760-4dfe-a1b6-5602fea86794
Version: 3
Effective Time: 20250531