EYE WASH- purified water liquid
Eye wash by
Drug Labeling and Warnings
Eye wash by is a Otc medication manufactured, distributed, or labeled by McKesson, Niagara Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
1 fl oz
NDC: 68599-1981-1
McKesson
Eye Wash
98.3% PURIFIED WATER
OPHTHALMIC SOLUTION | STERILEMFR# MCK19828
1 fl oz
(29.6 mL)Distributed by
McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0221
Made in Canada
1 fl oz eye wash solution
Drug Facts
Active ingredient Purpose
Purified water 98.3% Eyewash
Use
For cleansing the eye to remove loose foreign material
Warnings
For external use only
Do not use
if you experience any open wounds in or near the eyes and get medical help right away
if solution changes color or becomes cloudy
When using this product
to avoid contamination, do not touch tip of container to any surface
do not reuse
once opened, discard
Stop use and ask a doctor if you experience
eye pain
changes in vision
continued redness or irritation of the eye
condition worsens or persists
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
do not use if this seal is missing or broken
use before expiration date marked on bottle
lot number is printed on the bottle
store at 20º to 25ºC (68º to 77ºF)
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions? Call 1-800-777-4908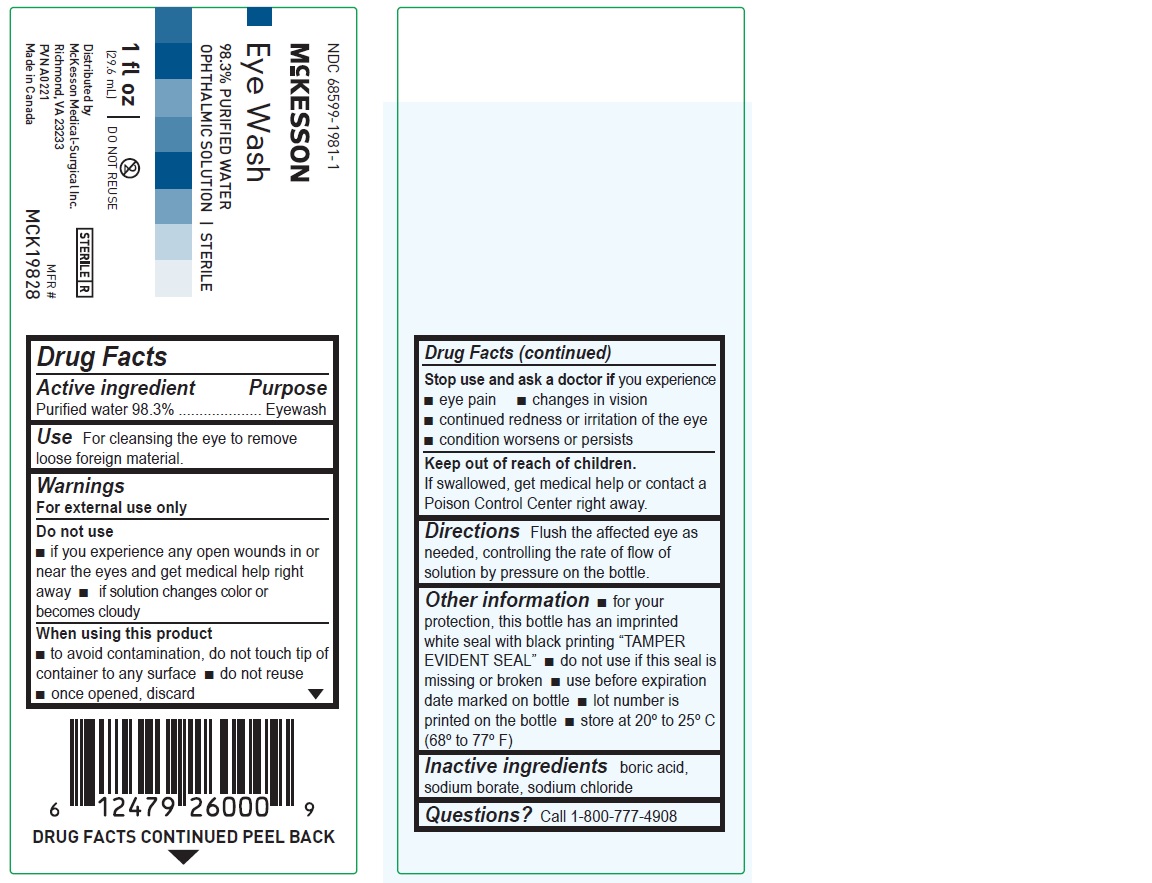

-
4 fl oz
68599-1981-4
McKesson
Eye Wash
98.3% PURIFIED WATER
OPHTHALMIC SOLUTION | STERILEMFR# MCK19818
4fl oz
(118.3mL)
Distributed by
McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0221
Made in Canada
4 fl oz eye wash solution
Drug Facts
Drug Facts
Active ingredient Purpose
Purified water 98.3% Eyewash
Use
For cleansing the eye to help relieve irritation or burning by removing loose foreign material
Warnings
For external use only
Do not use
if you experience any open wounds in or near the eyes and obtain immediate medical treatment
if solution changes color or becomes cloudy
When using this product
to avoid contamination, do not touch tip of container to any surface
do not reuse
once opened, discard
Stop use and ask a doctor if you experience
changes in vision
eye pain
condition worsens or persists
continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
lot number is printed on the bottle
store at 20º to 25ºC (68º to 77ºF)
for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
do not use if this seal is missing or broken
use before expiration date marked on bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions? Call 1-800-777-4908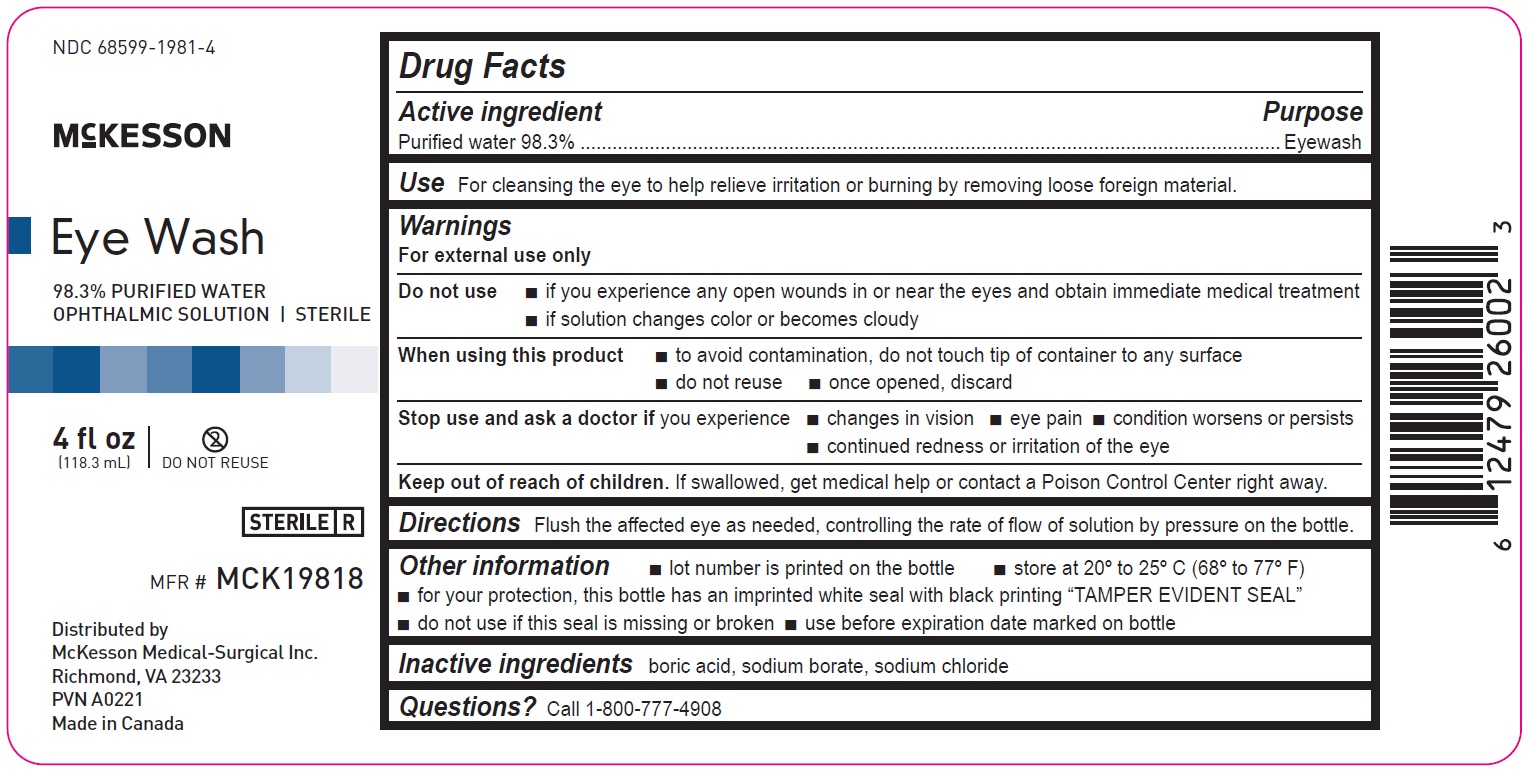

-
INGREDIENTS AND APPEARANCE
EYE WASH
purified water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68599-1981 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.3 mL in 100 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68599-1981-1 29.6 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/2021 2 NDC: 68599-1981-4 118.3 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 04/01/2021 Labeler - McKesson (023904428) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals 205477792 manufacture(68599-1981)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.