ACETAMINOPHEN tablet, film coated
Acetaminophen by
Drug Labeling and Warnings
Acetaminophen by is a Otc medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Elysium Pharmaceuticals Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
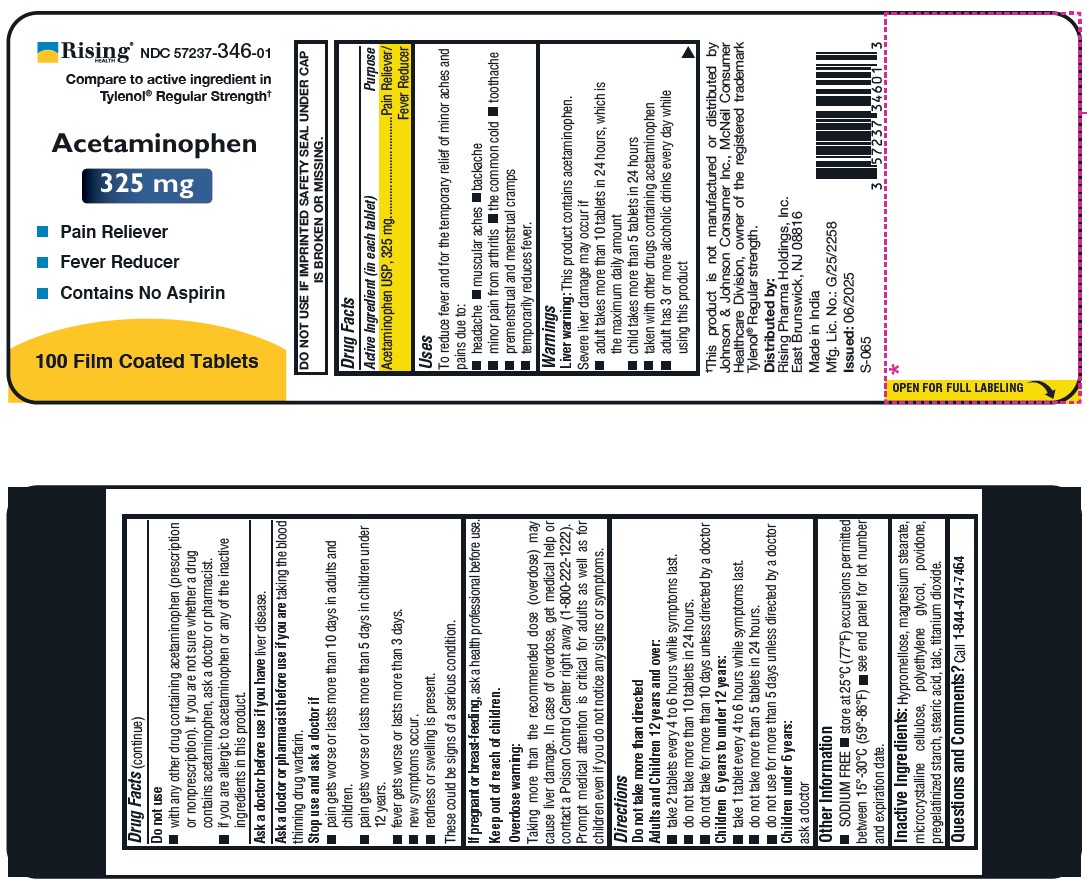
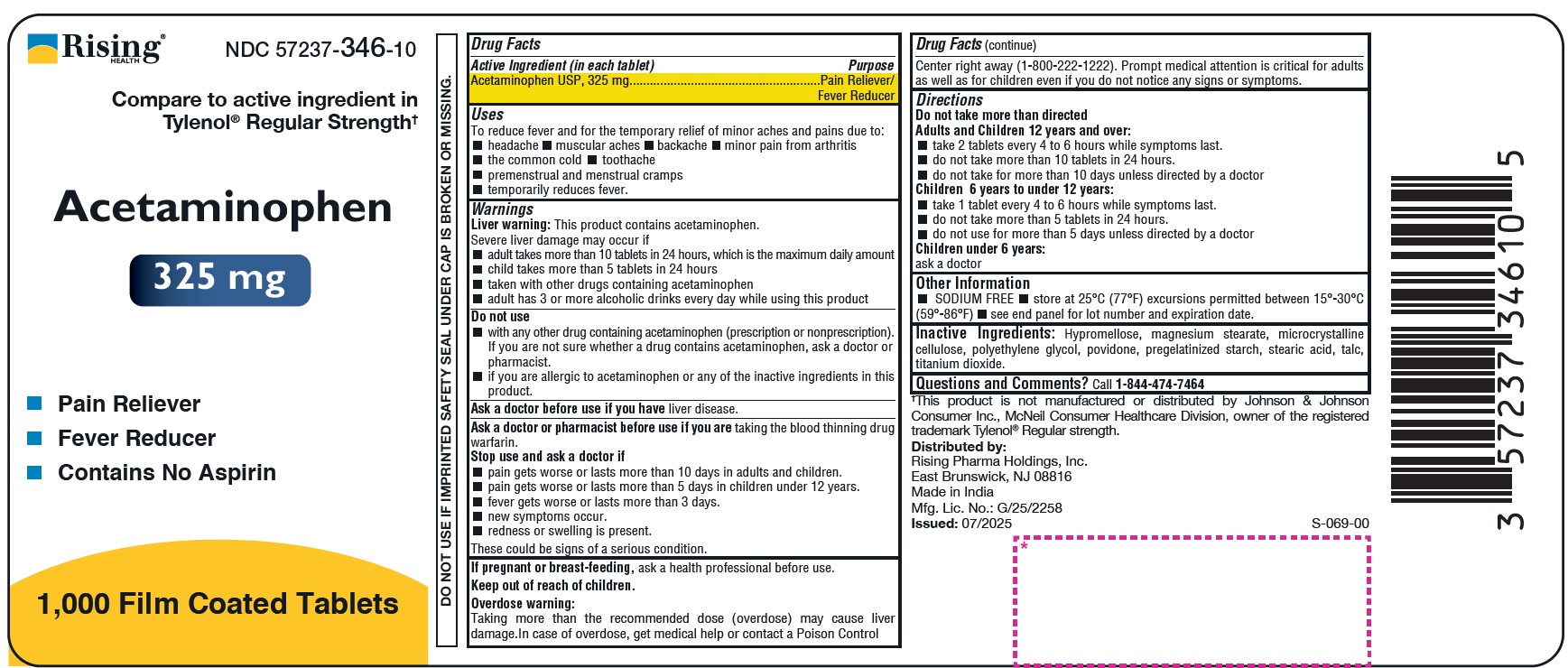
- DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING. Drug Facts
- PURPOSE
- Uses
-
Warnings
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if
- adult takes more than 10 tablets in 24 hours, which is the maximum daily amount
- child takes more than 5 tablets in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
- Do not use
- ASK DOCTOR
-
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin.
Stop use and ask a doctor if
pain gets worse or lasts more than 10 days in adults and children.
pain gets worse or lasts more than 5 days in children under 12 years.
fever gets worse or lasts more than 3 days.
new symptoms occur.
redness or swelling is present.
These could be signs of a serious condition. - PREGNANCY OR BREAST FEEDING
-
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
Overdose warning:
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. -
Directions
Do not take more than directed
Adults and Children 12 years and over:- take 2 tablets every 4 to 6 hours while symptoms last.
- do not take more than 10 tablets in 24 hours.
- do not take for more than 10 days unless directed by a doctor
Children 6 years to under 12 years:
- take 1 tablet every 4 to 6 hours while symptoms last.
- do not take more than 5 tablets in 24 hours.
- do not use for more than 5 days unless directed by a doctor
Children under 6 years:
ask a doctorOther Information
- SODIUM FREE
- store at 25°C (77°F) excursions permitted between 15°-30°C (59°-86°F)
- see end panel for lot number and expiration date.
- Inactive Ingredients
-
QUESTIONS
Questions and Comments? Call 1-844-474-7464
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
Mfg. Lic. No.: G/25/2258
Issued: 06/2025 (S-065)
Issued: 07/2025 (S-069-00)
†This product is not manufactured or distributed by Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division, owner of the registered trademark Tylenol® Regular strength.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57237-346 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (Off White) Score no score Shape ROUND Size 10mm Flavor Imprint Code S99 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57237-346-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2025 2 NDC: 57237-346-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 07/09/2025 Labeler - Rising Pharma Holdings, Inc. (116880195) Registrant - Elysium Pharmaceuticals Ltd (863182240) Establishment Name Address ID/FEI Business Operations Elysium Pharmaceuticals Ltd 863182240 analysis(57237-346) , label(57237-346) , manufacture(57237-346) , pack(57237-346)
Trademark Results [Acetaminophen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.