Body Conditioner by Make2Give LLC Body Conditioner
Body Conditioner by
Drug Labeling and Warnings
Body Conditioner by is a Otc medication manufactured, distributed, or labeled by Make2Give LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
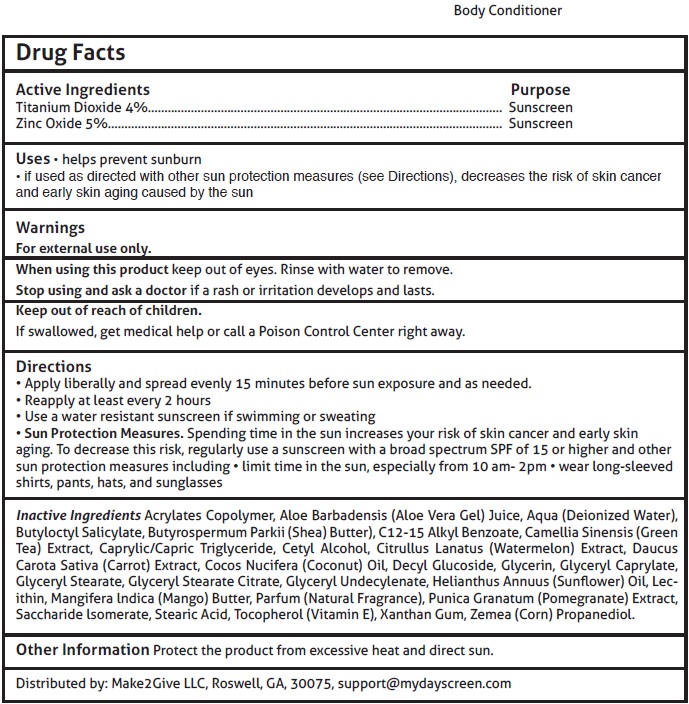
BODY CONDITIONER- titanium dioxide, zinc oxide cream
Make2Give LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Body Conditioner
Uses
helps prevent sunburn
if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
Apply liberally and spread evenly 15 minutes before sun exposure and as needed.
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including limit time in the sun, especially from 10 am- 2pm wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
Acrylates Copolymer, Aloe Barbadensis (Aloe Vera Gel) Juice, Aqua (Deionized Water), Butyloctyl Salicylate, Butyrospermum Parkii (Shea) Butter), C12-15 Alkyl Benzoate, Camellia Sinensis (Green Tea) Extract, Caprylic/Capric Triglyceride, Cetyl Alcohol, Citrullus Lanatus (Watermelon) Extract, Daucus Carota Sativa (Carrot) Extract, Cocos Nucifera (Coconut) Oil, Decyl Glucoside, Glycerin, Glyceryl Caprylate, Glyceryl Stearate, Glyceryl Stearate Citrate, Glyceryl Undecylenate, Helianthus Annuus (Sunflower) Oil, Lecithin, Mangifera lndica (Mango) Butter, Parfum (Natural Fragrance), Punica Granatum (Pomegranate) Extract, Saccharide lsomerate, Stearic Acid, Tocopherol (Vitamin E), Xanthan Gum, Zemea (Corn) Propanediol.
| BODY CONDITIONER
titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Make2Give LLC (023910159) |
Trademark Results [Body Conditioner]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BODY CONDITIONER 90022314 not registered Live/Pending |
Teresa Rinne 2020-06-26 |
 BODY CONDITIONER 86209655 not registered Dead/Abandoned |
Lunada Biomedical 2014-03-03 |
 BODY CONDITIONER 74435968 not registered Dead/Abandoned |
KAEPA, INC. 1993-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.