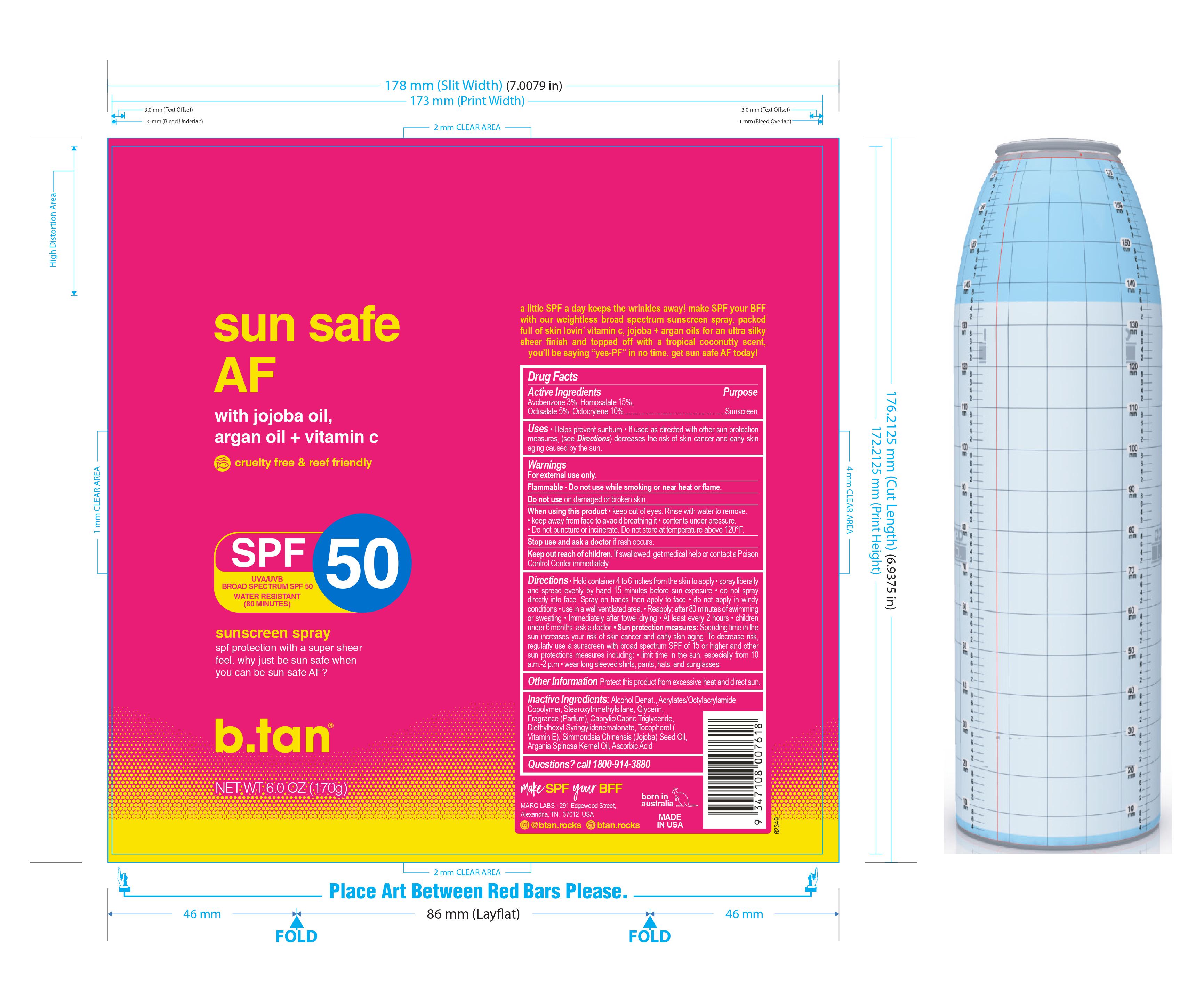
b.tan Sun Safe AF SPF 50 Spray
b.tan Sun Safe AF by
Drug Labeling and Warnings
b.tan Sun Safe AF by is a Otc medication manufactured, distributed, or labeled by Marque of Brands Americas, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
B.TAN SUN SAFE AF SPF 50- avobenzone, homosalate, octisalate, octocrylene spray
Marque of Brands Americas, LLC
----------
b.tan Sun Safe AF SPF 50 Spray
- Helps prevent sunburn
- If used as directed with other sun protection measures, ( see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
For external use only
Flammable - Do not use while smoking or near heat or flame.
Directions
- Hold container 4 to 6 inches from the skin to apply spray liberally and spread evenly by hand 15 minutes before sun exposure do not spray directly into face. Spray on hands then apply to face do not apply in windy conditions use in a well ventilated area. Reapply: after 80 minutes of swimming or sweating Immediately after towel drying At least every 2 hours children under 6 months: ask a doctor. Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m.-2 p.m wear long sleeved shirts, pants, hats, and sunglasses.
| B.TAN SUN SAFE AF
SPF 50
avobenzone, homosalate, octisalate, octocrylene spray |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Marque of Brands Americas, LLC (118733944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Marque of Brands Americas, LLC | 118733944 | manufacture(73978-007) | |
Revised: 8/2025
Document Id: 3d472fbf-7b8d-2717-e063-6394a90a19c7
Set id: bdbbffee-7747-7eb6-e053-2a95a90a3325
Version: 4
Effective Time: 20250826