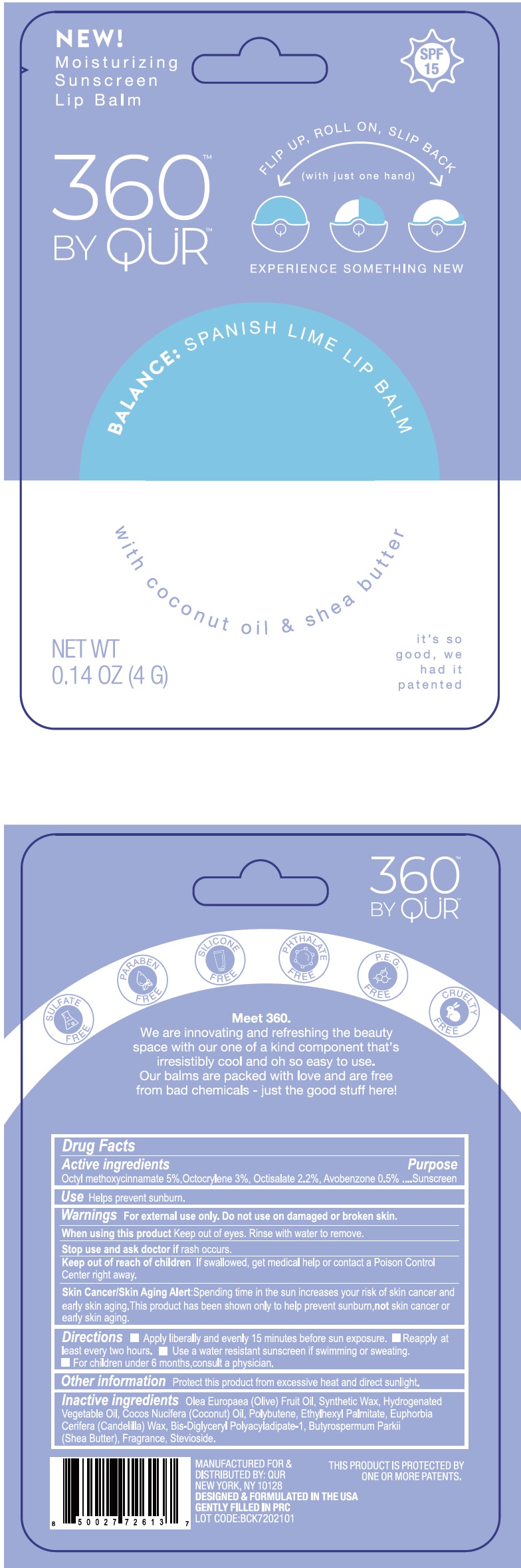
360 by QUR Spanish lime lip balm
360 by QUR Spanish lime lip balm by
Drug Labeling and Warnings
360 by QUR Spanish lime lip balm by is a Otc medication manufactured, distributed, or labeled by Bath Concept Cosmetics (Dongguan) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
360 BY QUR SPANISH LIME LIP BALM- octinoxate, octocrylene, octisalate, avobenzone stick
Bath Concept Cosmetics (Dongguan) Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
360 by QUR Spanish lime lip balm
Warnings
For external use only.
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Skin Cancer/Skin Aging Alert: Spending time in the sun increase your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every two hours.
- Use a water resistant sunscreen if swimming or sweating.
- For children under 6 months, consult a physician.
| 360 BY QUR SPANISH LIME LIP BALM
octinoxate, octocrylene, octisalate, avobenzone stick |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bath Concept Cosmetics (Dongguan) Co., Ltd (529623933) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.