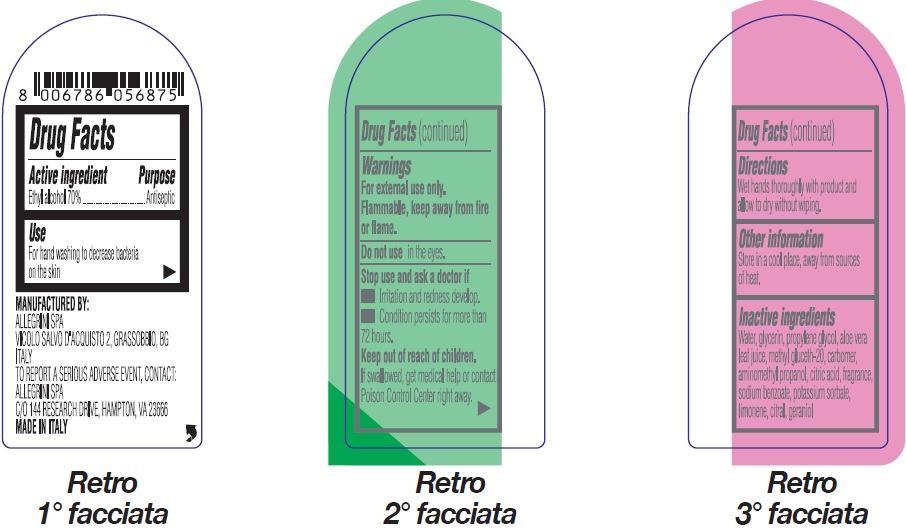
Primagel Plus Rinse-Free Antiseptic Hand Sanitizer 70% Ethyl Alcohol
Primagel Plus Rinse-Free Antiseptic Hand Sanitizer 70 Ethyl Alcohol by
Drug Labeling and Warnings
Primagel Plus Rinse-Free Antiseptic Hand Sanitizer 70 Ethyl Alcohol by is a Otc medication manufactured, distributed, or labeled by Allegrini SPA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PRIMAGEL PLUS RINSE-FREE ANTISEPTIC HAND SANITIZER 70 ETHYL ALCOHOL- alcohol gel
Allegrini SPA
----------
Primagel Plus Rinse-Free Antiseptic Hand Sanitizer 70% Ethyl Alcohol
Warnings
For external use only.
Flammable, keep away from fire or flame.
| PRIMAGEL PLUS RINSE-FREE ANTISEPTIC HAND SANITIZER 70 ETHYL ALCOHOL
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Allegrini SPA (429407935) |
Revised: 1/2024
Document Id: 0dfc2e0c-856c-e678-e063-6294a90a1c08
Set id: be4dc6f3-c741-eb9f-e053-2a95a90ab4e4
Version: 3
Effective Time: 20240102
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.