LEVOCETIRIZINE DIHYDROCHLORIDE tablet, film coated
Levocetirizine Dihydrochloride by
Drug Labeling and Warnings
Levocetirizine Dihydrochloride by is a Otc medication manufactured, distributed, or labeled by Camber Consumer Care, Hetero Labs Limited Unit III. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
adults 65 years of age and older
- ask a doctor
adults and children 12 to 64 years of age
- take 1 tablet (5 mg) once daily in the evening
- do not take more than 1 tablet (5 mg) in 24 hours
- ½ tablet (2.5 mg) once daily in the evening may be appropriate for less severe symptoms
children 6 to 11 years of age
- take ½ tablet (2.5 mg) once daily in the evening
- do not take more than ½ tablet (2.5 mg) in 24 hours
children under 6 years of age
- do not use
consumers with kidney disease
- do not use
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS or COMMENTS?
-
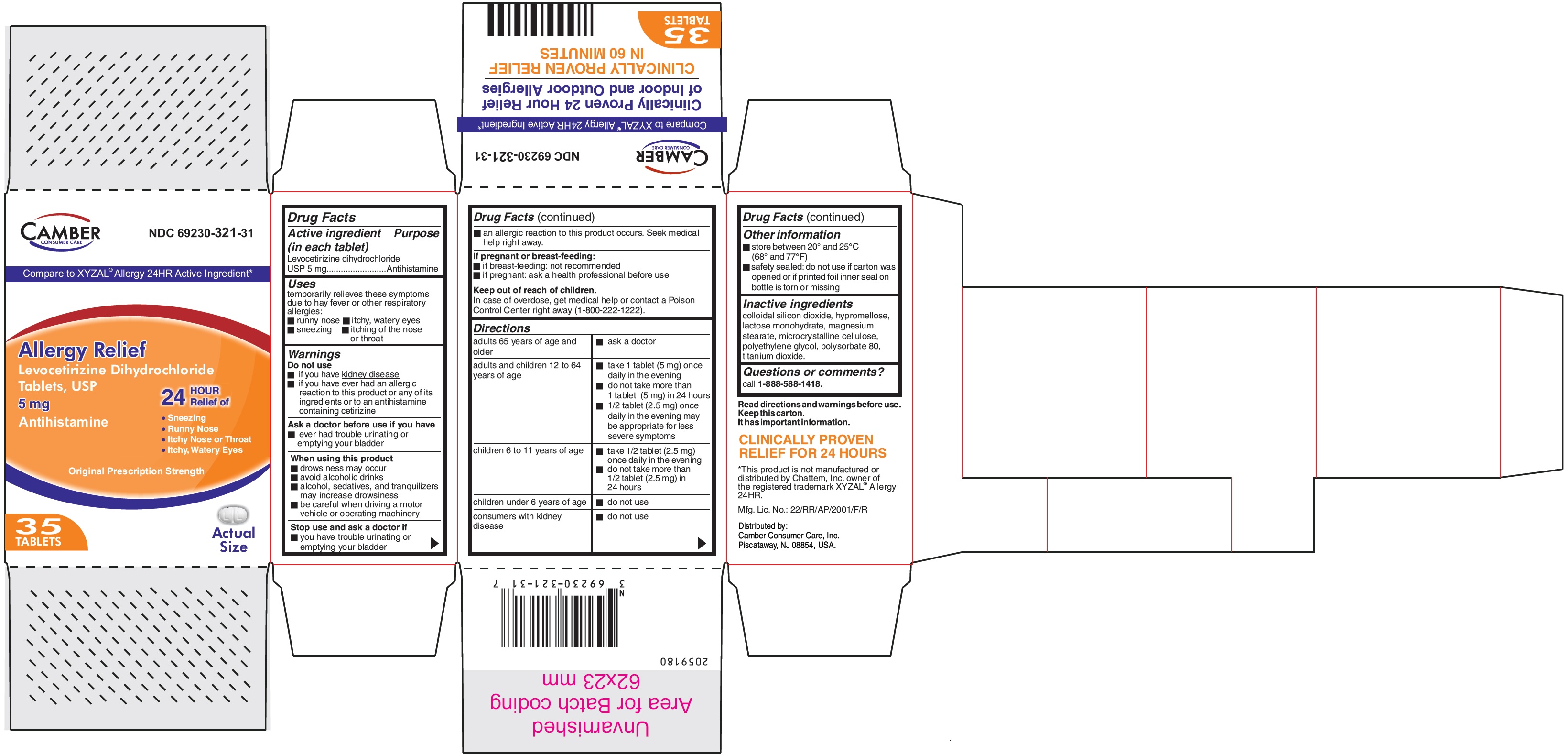
PRINCIPAL DISPLAY PANEL
Levocetirizine Dihydrochloride Tablets USP 5 mg-35's Container carton
Compare to XYZAL® Allergy 24HR Active Ingredient*Allergy Relief
Levocetirizine Dihydrochloride
Tablets, USP5 mg
Antihistamine
24 HOUR Relief of
- Sneezing
- Runny Nose
- Itchy Nose or Throat
- Itchy, Watery Eyes
Original Prescription Strength
35 TABLETS
Levocetirizine Dihydrochloride Tablets USP 5 mg - 35's Container label
Compare to XYZAL® Allergy 24HR Active Ingredient*
Allergy Relief
Levocetirizine Dihydrochloride
Tablets, USP5 mg
Antihistamine
24 HOUR Relief of
Sneezing
Runny Nose
Itchy Nose or Throat
Itchy, Watery EyesOriginal Prescription Strength
35 TABLETS

-
INGREDIENTS AND APPEARANCE
LEVOCETIRIZINE DIHYDROCHLORIDE
levocetirizine dihydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69230-321 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCETIRIZINE DIHYDROCHLORIDE (UNII: SOD6A38AGA) (LEVOCETIRIZINE - UNII:6U5EA9RT2O) LEVOCETIRIZINE DIHYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE (White to off white) Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code H;LL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69230-321-31 1 in 1 CARTON 10/28/2020 1 35 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 69230-321-32 1 in 1 CARTON 10/28/2020 2 55 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 69230-321-33 1 in 1 CARTON 10/28/2020 3 80 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 69230-321-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2020 5 NDC: 69230-321-01 10 in 1 CARTON 10/28/2020 5 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC: 69230-321-34 1 in 1 CARTON 10/28/2020 6 180 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213513 10/28/2020 Labeler - Camber Consumer Care Inc (079539968) Establishment Name Address ID/FEI Business Operations Hetero Labs Limited Unit III 676162024 ANALYSIS(69230-321) , MANUFACTURE(69230-321)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.