proctofoam® non-steroid (pramoxine hydrochloride 1%)
Proctofoam by
Drug Labeling and Warnings
Proctofoam by is a Otc medication manufactured, distributed, or labeled by Alaven Pharmaceutical LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROCTOFOAM- pramoxine hydrochloride aerosol, foam
Alaven Pharmaceutical LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
proctofoam®
non-steroid
(pramoxine hydrochloride 1%)
Warnings
Do not exceed the recommended daily dosage unless directed by a physician.
If condition worsens or does not improve within 7 days, consult a physician.
In case of rectal bleeding, consult a physician promptly.
Do not put this product into the rectum by using fingers or any mechanical device or applicator. Do not insert any part of the aerosol container into the rectum.
Directions
- Place cap on container. Shake well before use.
- Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
- Gently dry by patting or blotting with toilet tissue or a soft cloth before application of proctofoam®.
- Dispense proctofoam® onto a clean tissue and apply externally to the affected area up to 5 times daily.
- Children under 12 years of age: consult a physician.
Other information
- Store upright at controlled room temperature 20° - 25°C (68° - 77°F); excursions permitted between 15° - 30°C (59° - 86°F).
- Do not refrigerate.
- Contents of the container are under pressure. Do not burn or puncture the aerosol container.
- Do not store at temperatures above 120°F (49°C).
Inactive ingredients
cetyl alcohol, glyceryl monostearate and PEG-100 stearate blend, methylparaben, polyoxyethylene 23 lauryl ether, polyoxyl 40 stearate, propylene glycol, propylparaben, purified water, trolamine and inert propellants: isobutane and propane.
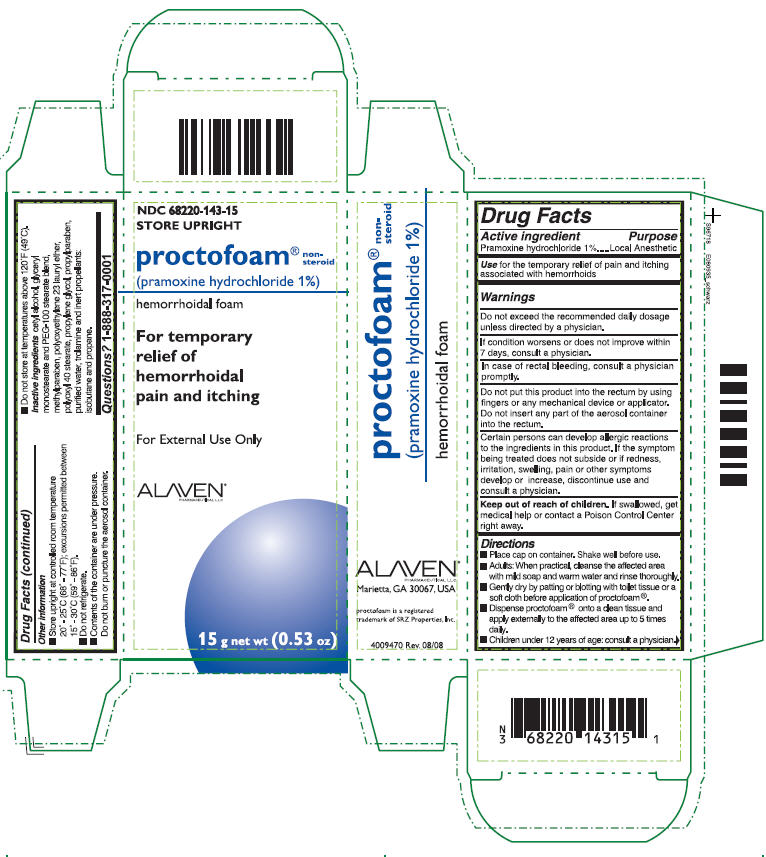
PRINCIPAL DISPLAY PANEL - 15 g Carton
NDC: 68220-143-15
STORE UPRIGHT
proctofoam®
non-
steroid
(pramoxine hydrochloride 1%)
hemorrhoidal foam
For temporary
relief of
hemorrhoidal
pain and itching
For External Use Only
ALAVEN®
PHARMACEUTICAL LLC
15 g net wt (0.53 oz)
PRINCIPAL DISPLAY PANEL - 15 g Label
NDC: 68220-143-15
For External Use Only
proctofoam® non-steroid
(pramoxine hydrochloride 1%)
hemorrhoidal foam
DIRECTIONS: Place cap on container. Shake well before use. Apply externally to affected
area up to 5 times daily. See carton for additional directions for use.
WARNINGS: Do not exceed the recommended daily dosage unless directed by a
physician. If condition worsens or does not improve within 7 days, consult a physician. In
case of rectal bleeding, consult a physician promptly.
Do not put this product into the rectum by using fingers or any mechanical device or
applicator. Certain persons can develop allergic reactions to ingredients in this product. If
the symptom being treated does not subside or if redness, irritation, swelling, pain or other
symptoms develop or increase, discontinue use and consult a physician. Do not use in the
eyes or nose. Do not apply to large areas of the body.
CAUTION: Do not insert any part of the aerosol container into the rectum. Contents of
the container are under pressure. Do not burn or puncture the aerosol container. Do not
store at temperatures above 120°F (49°C).
STORE UPRIGHT AT CONTROLLED ROOM TEMPERATURE 20° - 25°C (68° -
77°F); excursions permitted between 15° - 30°C (59° - 86°F).
DO NOT REFRIGERATE.
Keep this and all drugs out of the reach of children. In case of accidental
ingestion, seek professional assistance or contact a Poison Control Center
immediately.
ALAVEN®
PHARMACEUTICAL LLC
Marietta, GA 30067, USA
For medical inquiries, call 1-888-317-0001
15 g net weight (0.53 oz)
Rev. 05/08
| PROCTOFOAM
pramoxine hydrochloride aerosol, foam |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Alaven Pharmaceutical LLC (140210829) |