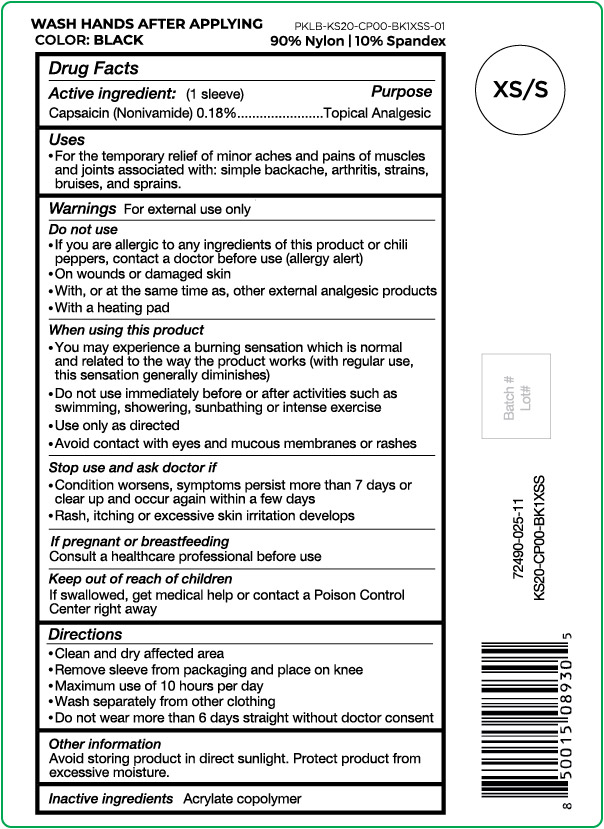
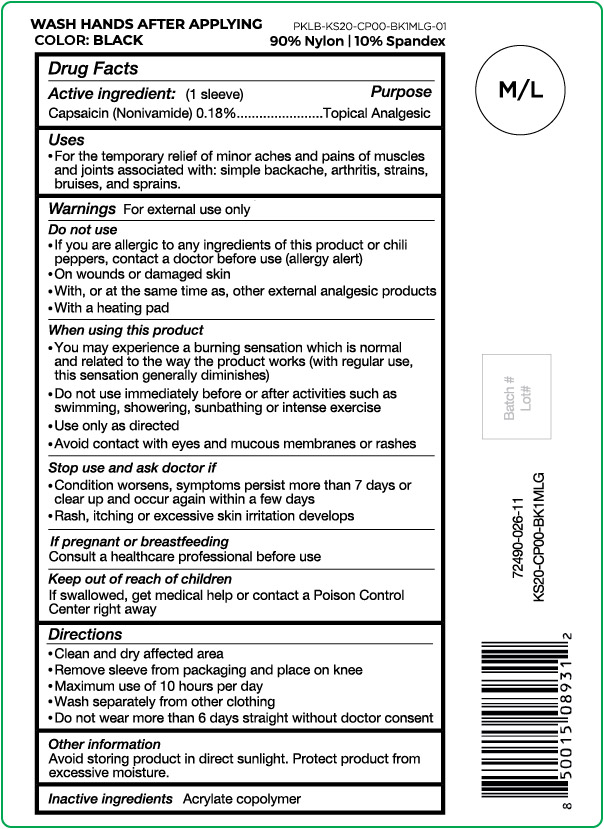
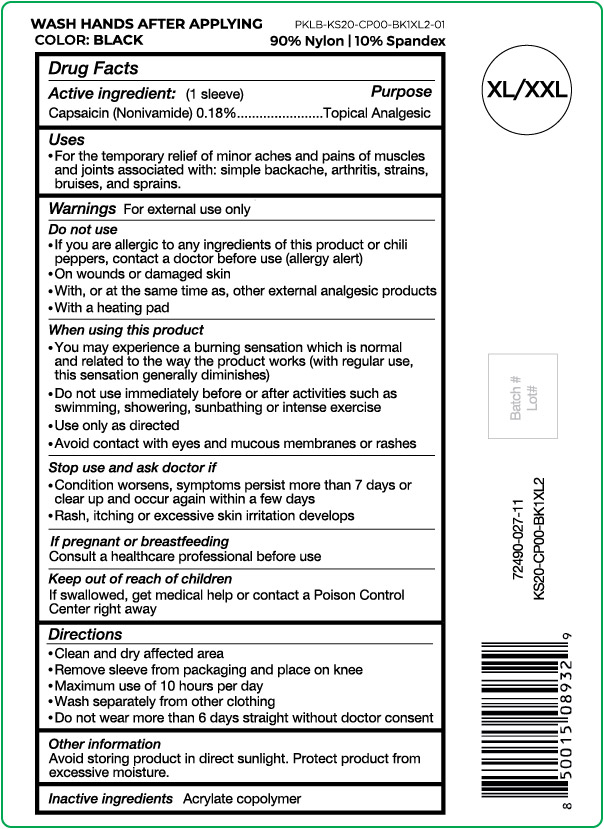
Active ingredient
Capsaicin (Nonivamide) 0.18%
Purpose
Topical Analgesic
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with: simple backache, arthritis, strains, bruises and sprains.
Warnings
For external use only
Do not use
- If you are allergic to any ingredients of this product or chili peppers, contact a doctor before use (allergy alert)
- On wounds or damaged skin
- With, or at the same time as, other external analgesic products
- With a heating pad
When using this product
- You may experience a burning sensation which is normal and related to the way the product works (with regular use, this sensation generally diminishes)
- Do not use immediately before or after activities such as swimming, showering, sunbathing or intense exercise
- Use only as directed
- Avoid contact with eyes and mucous membranes or rashes
Stop use and ask doctor if
- Condition worsens, symptoms persist more than 7 days or clear up and occur again within a few days
- Rash, itching or excessive skin irritation develops
If pregnant or breast feeding
Consult a healthcare professional before use
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Clean and dry affected area
- Remove sleeve from packaging and place on knee
- Maximum use of 10 hours per day
- Wash separately from other clothing
- Do not wear more than 6 days straight without doctor consent
Other information
Avoid storing product in direct sunlight. Protect product from excessive moisture.
Inactive ingredients
Acrylate copolymer