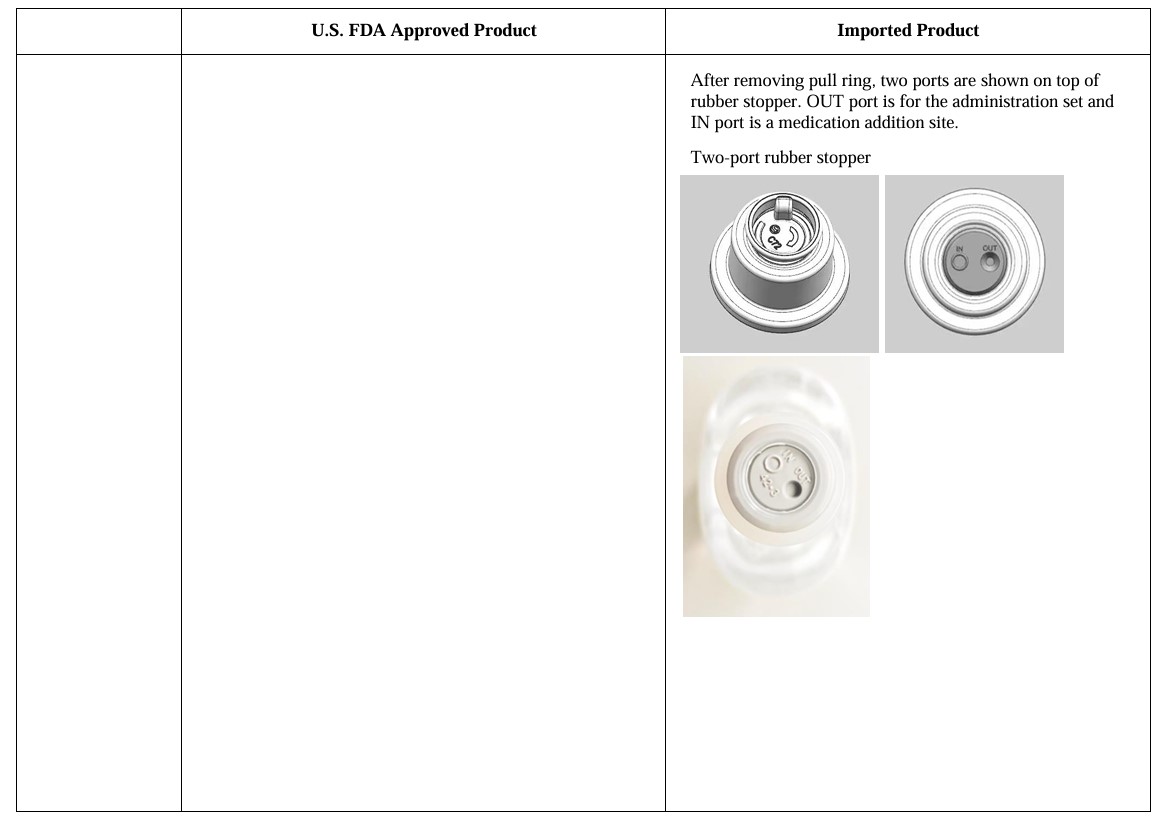
0.9% Sodium Chloride Injection in UNIFLEX Plastic Container
Sodium Chloride by
Drug Labeling and Warnings
Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by ApiJect Systems Corp, Sichuan Kelun Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SODIUM CHLORIDE- sodium chloride injection, solution
ApiJect Systems Corp
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
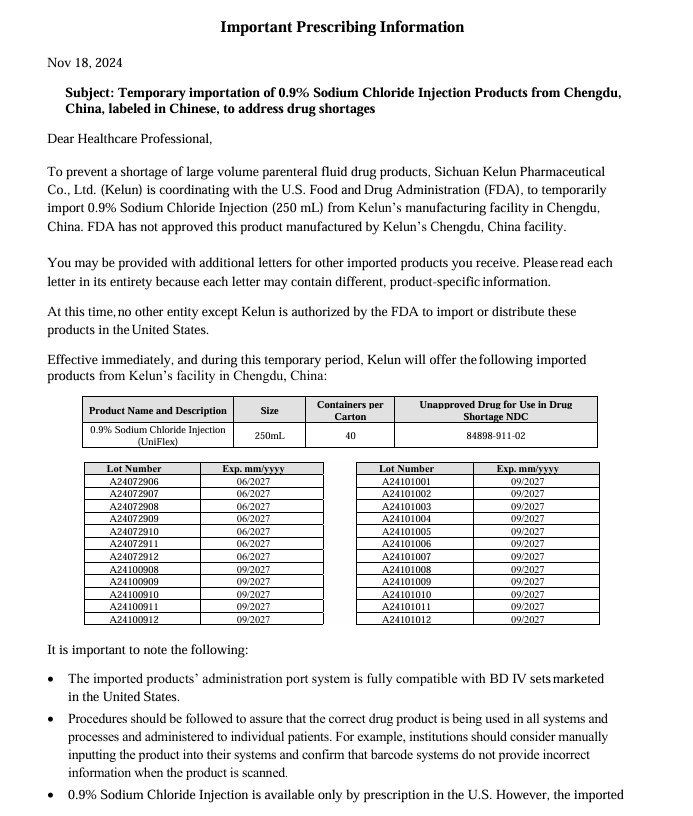
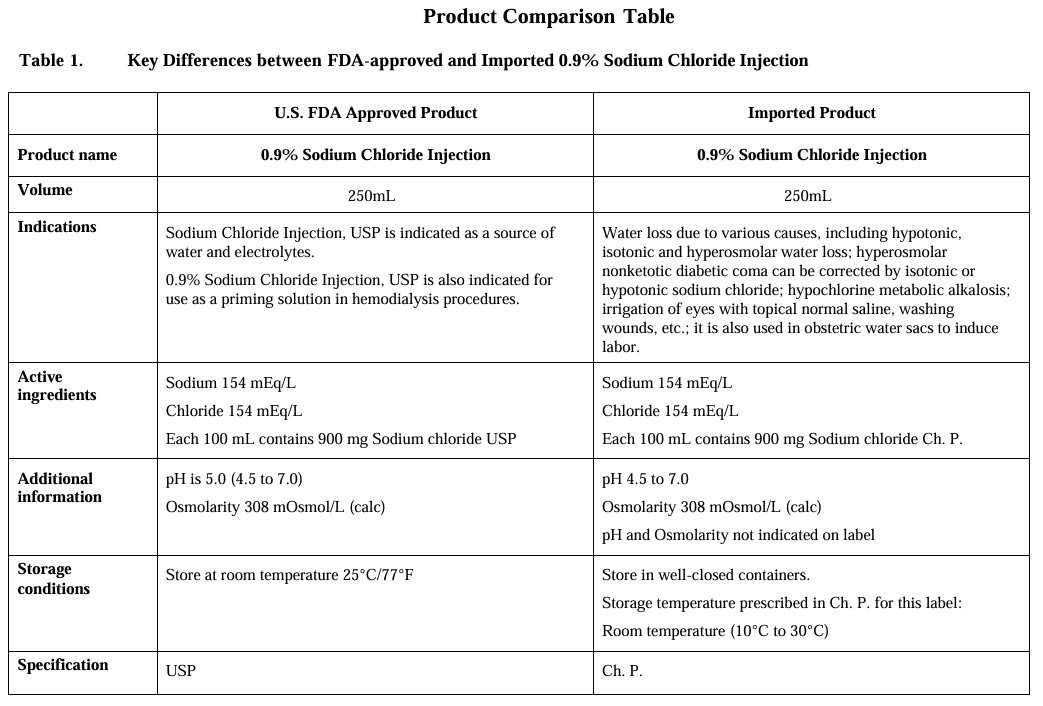
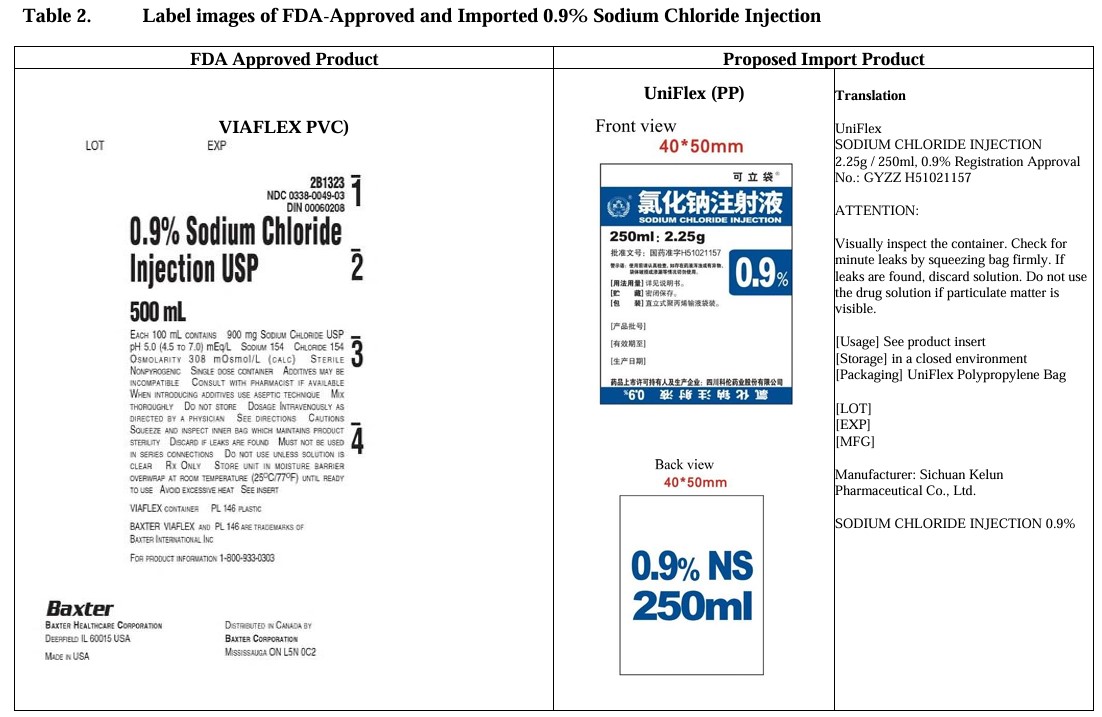
0.9% Sodium Chloride Injection
in UNIFLEX Plastic Container
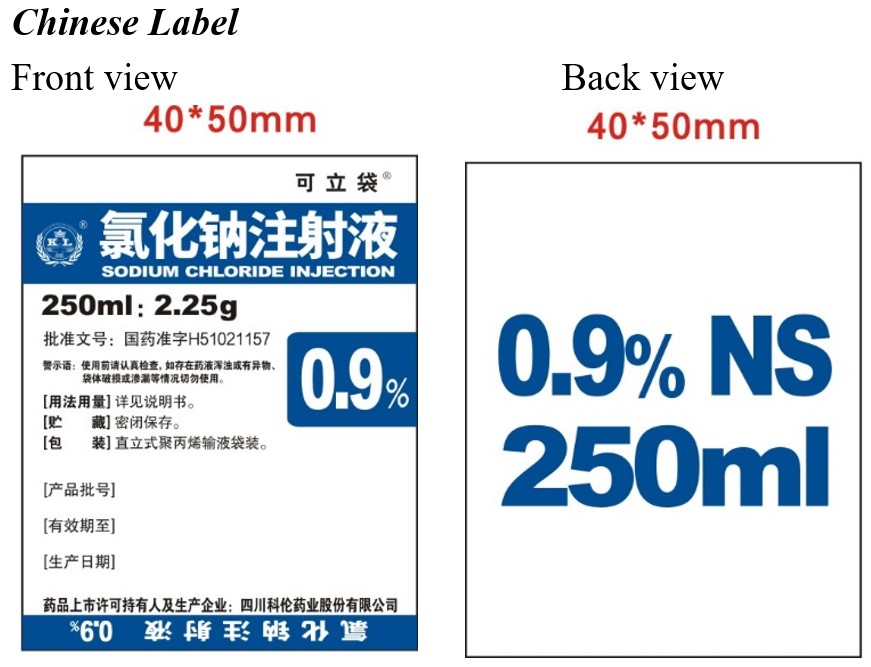
Translation:
UniFlex
SODIUM CHLORIDE INJECTION
2.25g / 250ml 0.9%
Registration Approval No.: GYZZ H51021157
ATTENTION:
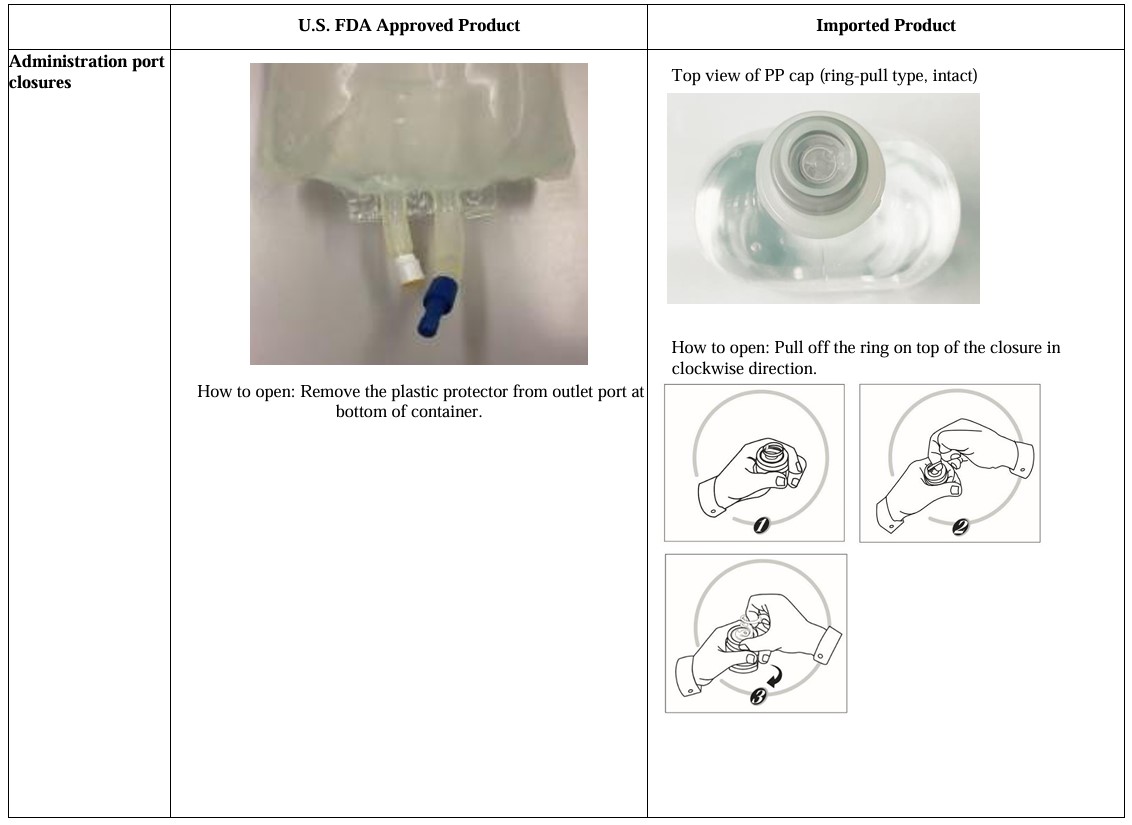
Visually inspect the container. Check for minute leaks by squeezing bag firmly.
If leaks are found, discard solution. Do not use the drug solution if particulate matter is visible.
[Usage] See product insert
[Storage] in a closed environment
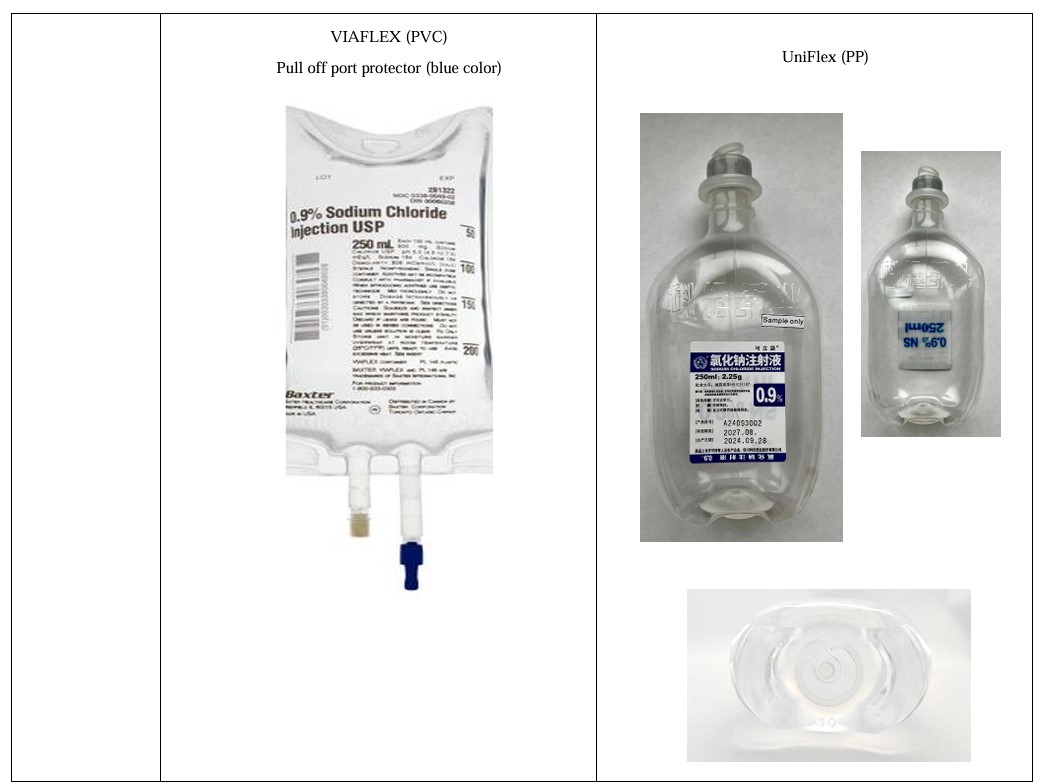
[Packaging] UniFlex Polypropylene Bag
[LOT]
[EXP]
[MFG]
Manufacturer:
Sichuan Kelun Pharmaceutical Co., Ltd.

Translation:
| Do not open with sharp object.
Do not stack higher than 12 layers. | Qualification printed on flap
Qualification For any quality issues or quantitative insufficiencies, please contact: Hotline: 4006860333, from Monday to Friday: 08:00-17:30, excluding official holidays. Company Website: www.kelun.com | Store as per storage requirements to ensure quality of drug products. | |
| UniFlex Polypropylene Bag
SODIUM CHLORIDE INJECTION 0.9%
250ml Product Information LOT
Sichuan Kelun Pharmaceutical Co., Ltd. | 250ml
Stack upwards, Protect from moisture and light Authorized Holder:
Sichuan Kelun Pharmaceutical Co., Ltd. | UniFlex Polypropylene Bag
SODIUM CHLORIDE INJECTION 0.9%
250ml Product Information LOT
Sichuan Kelun Pharmaceutical Co., Ltd. | 250ml
Stack upwards, Protect from moisture and light Product name: SODIUM CHLORIDE INJECTION (0.9%)
Sichuan Kelun Pharmaceutical Co., Ltd. |

Size: 150mmⅹ70mm
SODIUM CHLORIDE INJECTION
UniFlex Plastic Container 250 mL
"Pack size: 40 containers/carton
LOT:
EXP:
MFG:"
Storage: Store at room temperature (10°C-30°C). Do not freeze.
"Imported by:
ApiJect Systems Corp.
2 High Ridge Park, Stamford, CT 06905 USA
Tel: 855-826-4885"
Manufactured by:
Sichuan Kelun Pharmaceutical Co., Ltd.
South of No.2 Road, Xindu Satellite City Industrial Development Zone, Chengdu, Sichuan, P.R.China
| SODIUM CHLORIDE
sodium chloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ApiJect Systems Corp (117008506) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sichuan Kelun Pharmaceutical Co., Ltd. | 529053803 | analysis(84898-911) , label(84898-911) , manufacture(84898-911) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.