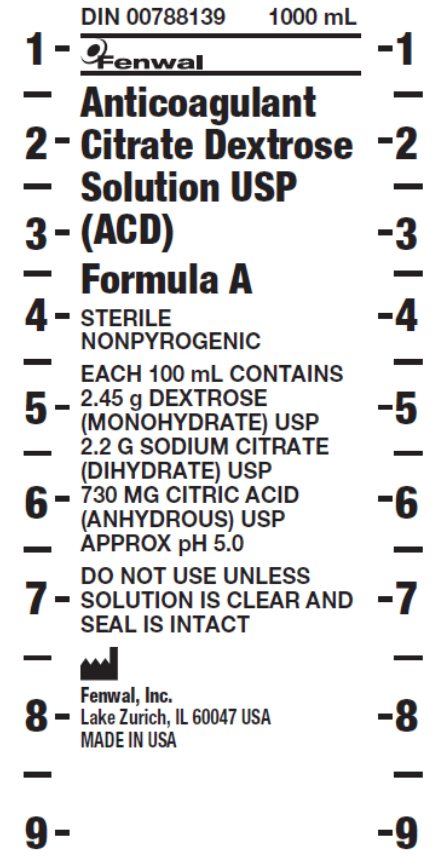
ACD-A- antiocoagulant citrate dextrose solution, formula a solution
ACD-A by
Drug Labeling and Warnings
ACD-A by is a Prescription medication manufactured, distributed, or labeled by Fenwal Inc., Fenwal International, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- WARNINGS
- HOW SUPPLIED
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACD-A
antiocoagulant citrate dextrose solution, formula a solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0942-9005 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.45 g in 100 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 2.2 g in 100 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 730 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0942-9005-01 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN160918 10/24/2012 Labeler - Fenwal, Inc. (794519020) Registrant - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-9005)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.