BAL- dimercaprol injection
BAL by
Drug Labeling and Warnings
BAL by is a Prescription medication manufactured, distributed, or labeled by Akorn, Akorn Operating Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
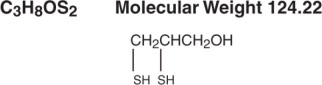
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
The sulfhydryl groups of dimercaprol form complexes with certain heavy metals thus preventing or reversing the metallic binding of sulfhydryl-containing enzymes. The complex is excreted. The sustained presence of dimercaprol promotes continued excretion of the metallic poisons - arsenic, gold and mercury. It is also used in combination with Edetate Calcium Disodium Injection USP to promote the excretion of lead.
-
INDICATIONS
BAL in Oil (Dimercaprol Injection USP) is indicated in the treatment of arsenic, gold and mercury poisoning. It is indicated in acute lead poisoning when used concomitantly with Edetate Calcium Disodium Injection USP.
Dimercaprol Injection USP is effective for use in acute poisoning by mercury salts if therapy is begun within one or two hours following ingestion. It is not very effective for chronic mercury poisoning.
Dimercaprol Injection USP is of questionable value in poisoning caused by other heavy metals such as antimony and bismuth. It should not be used in iron, cadmium, or selenium poisoning because the resulting dimercaprol-metal complexes are more toxic than the metal alone, especially to the kidneys.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Because the dimercaprol-metal complex breaks down easily in an acid medium, production of an alkaline urine affords protection to the kidney during therapy. Medicinal iron should not be administered to patients under therapy with BAL in Oil (Dimercaprol Injection USP).
BAL in Oil Ampules is formulated with peanut oil. Peanut oil may cause allergic reactions in some individuals. Physicians should use caution in prescribing BAL in Oil Ampules for peanut-sensitive patients. Medication and equipment necessary to treat allergic reactions should be available if the product is administered to peanut-allergic patients.
Pregnancy Category C
Animal reproduction studies have not been conducted with BAL in Oil. It is also not known whether BAL in Oil can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. BAL in Oil should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. However, because many drugs are excreted in human milk, caution should be exercised when BAL in Oil is administered to a nursing woman.
-
ADVERSE REACTIONS
One of the most consistent responses to Dimercaprol Injection USP is a rise in blood pressure accompanied by tachycardia. This rise is roughly proportional to the dose administered. Doses larger than those recommended may cause other transitory signs and symptoms in approximate order of frequency as follows: (1) nausea and, in some instance, vomiting; (2) headache; (3) a burning sensation in the lips, mouth and throat; (4) a feeling of constriction, even pain, in the throat, chest, or hands; (5) conjunctivitis, lacrimation, blepharal spasm, rhinorrhea, and salivation; (6) tingling of the hands; (7) a burning sensation in the penis; (8) sweating of the forehead, hands and other areas; (9) abdominal pain; and (10) occasional appearance of painful sterile abscesses. Many of the above symptoms are accompanied by a feeling of anxiety, weakness, and unrest and often are relieved by administration of antihistamine.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSE
-
DOSAGE AND ADMINISTRATION
By deep intramuscular injection only. For mild arsenic or gold poisoning, 2.5 mg/kg of body weight four times daily for two days, two times on the third day, and once daily thereafter for ten days; for severe arsenic or gold poisoning, 3 mg/kg every four hours for two-days, four times on the third day, then twice daily thereafter for ten days. For mercury poisoning, 5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days. For acute lead encephalopathy, 4 mg/kg body weight is given alone in the first dose and thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site. For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose. Treatment is maintained for two to seven days depending on clinical response. Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals. Other supportive measures should always be used in conjunction with BAL in Oil (Dimercaprol Injection USP) therapy.
BAL in Oil should be inspected visually for particulate matter and discoloration prior to administration.
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-526-03
BAL in Oil
DIMERCAPROL INJECTION, USP
100 mg/mL
For IM Use Only
Rx only 3 mL Ampule
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-526-03
BAL in Oil
DIMERCAPROL INJECTION, USP
100 mg/mL
For Intramuscular Injection Only
10 Ampules (3 mL each)
Rx only Akorn Logo -
INGREDIENTS AND APPEARANCE
BAL
dimercaprol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-526 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimercaprol (UNII: 0CPP32S55X) (Dimercaprol - UNII:0CPP32S55X) Dimercaprol 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzyl Benzoate (UNII: N863NB338G) 200 mg in 1 mL Peanut Oil (UNII: 5TL50QU0W4) 700 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-526-03 10 in 1 CARTON 05/01/2012 1 3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA005939 05/01/2012 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 063434679 PACK(17478-526) , LABEL(17478-526) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-526) , ANALYSIS(17478-526) , STERILIZE(17478-526)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.