AC FAST- paracetamol capsule, coated
AC FAST by
Drug Labeling and Warnings
AC FAST by is a Otc medication manufactured, distributed, or labeled by GRIMANN SA DE CV. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
- PEDIATRIC USE
- PREGNANCY
- CONTRAINDICATIONS
- ADVERSE REACTIONS
- PURPOSE
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AC FAST
paracetamol capsule, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 81660-222 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg in 1 U Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) 54 mg in 1 U CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 19 mg in 1 U SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) 15 mg in 1 U MAGNESIUM STEARATE (UNII: 70097M6I30) 2 mg in 1 U POLYVINYL ALCOHOL/POLYVINYL ACETATE COPOLYMER (4:1; 9500 MW) (UNII: 2AVG41YG39) 12 mg in 1 U Product Characteristics Color white Score score with uneven pieces Shape OVAL Size 12mm Flavor Imprint Code 500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81660-222-01 10 U in 1 BLISTER PACK; Type 0: Not a Combination Product 04/01/2021 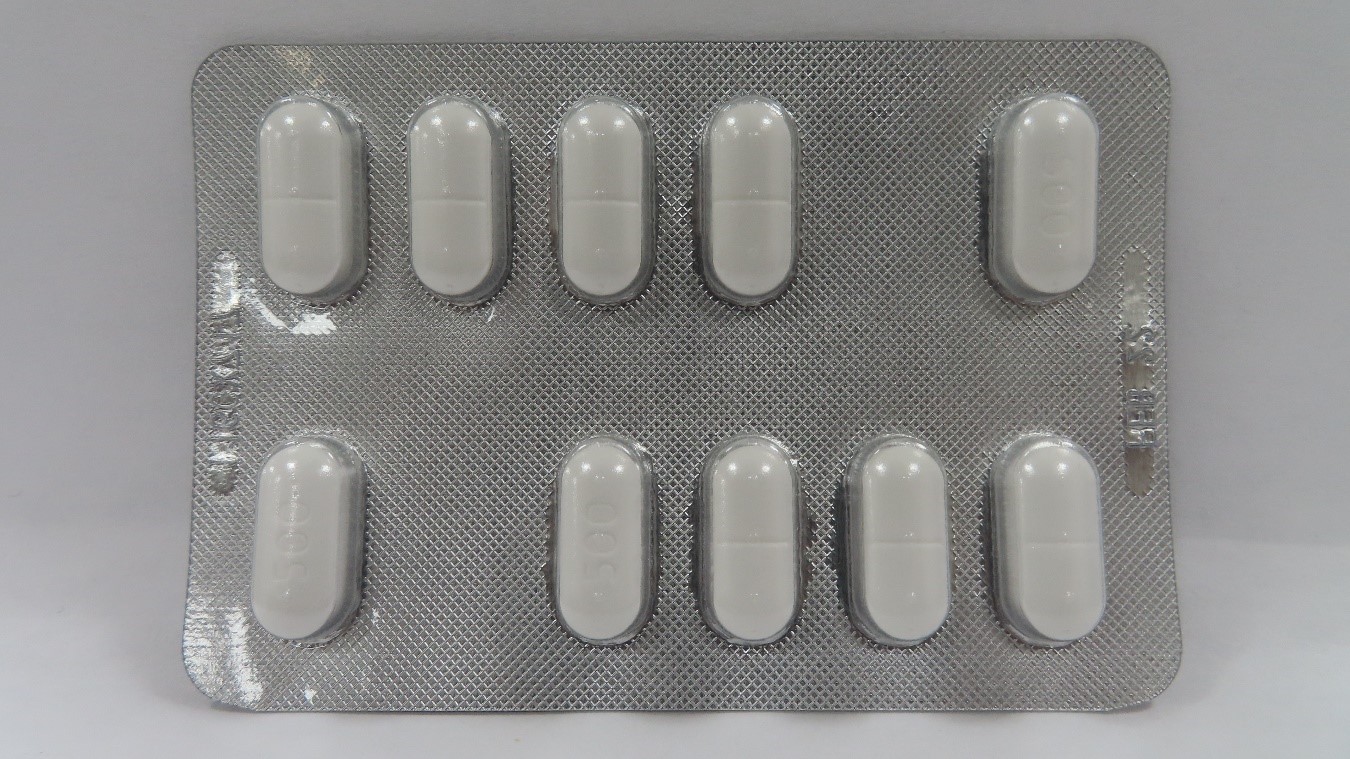
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/01/2021 Labeler - GRIMANN SA DE CV (812806982) Registrant - GRIMANN SA DE CV (812806982) Establishment Name Address ID/FEI Business Operations GRIMANN SA DE CV 812806982 manufacture(81660-222)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

