PRO-PEN-G- penicillin g procaine injection, suspension
Pro-Pen-G by
Drug Labeling and Warnings
Pro-Pen-G by is a Animal medication manufactured, distributed, or labeled by Bimeda, Inc., Constant Irwindale. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
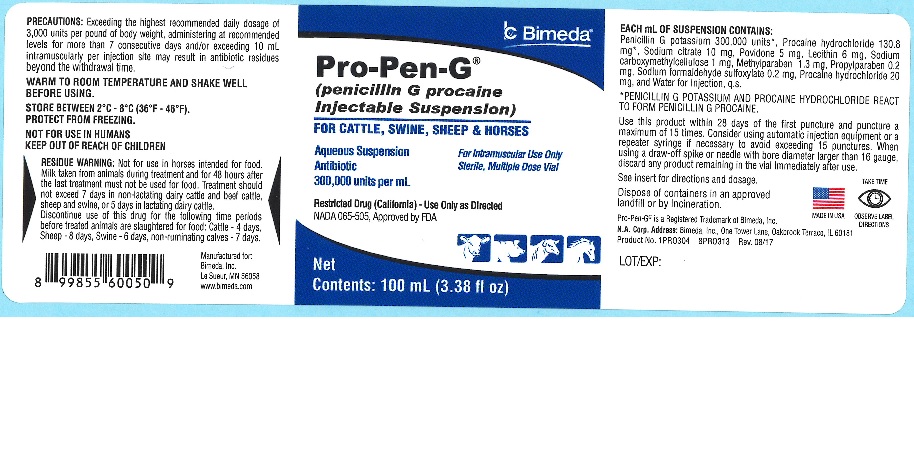
Pro-Pen-G®
(penicillin G procaine Injectable Suspension)
FOR CATTLE, SWINE, SHEEP & HORSES
Aqueous Suspension For Intramuscular Use Only
Antibiotic Sterile, Multiple Dose Vial
300,000 units per mL
Restricted Drug (California) - Use Only as Directed
NADA 065-505, Approved by FDA
NOT FOR USE IN HUMANS KEEP OUT OF REACH OF CHILDREN
DESCRIPTION:
Penicillin G Procaine Injectable Suspension Alternative Method is available in 100-mL, 250-mL and 500-mL multiple-dose vials. Each mL contains: Active Ingredients: Penicillin G Procaine 300,000 units*, Procaine hydrochloride 130.8 mg*. Inactive Ingredients: Methylparaben 1.3 mg, Propylparaben 0.2 mg, Sodium citrate 10 mg, Sodium carboxymethylcellulose 1 mg, Povidone 5 mg, Lecithin 6 mg, Sodium formaldehyde sulfoxylate 0.2 mg, Procaine hydrochloride 20 mg, and Water for injection, q.s.
*Penicillin G Potassium and Procaine Hydrochloride react to form Penicillin G Procaine.
- MECHANISM OF ACTION
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
DIRECTIONS FOR USE: The suspension should be administered by deep intramuscular injection within the fleshy muscles of the hip, rump, round or thigh, or into the neck, changing the site for each injection. Do not inject subcutaneously, into a blood vessel, or near a major nerve. Use a 16 or 18 gauge needle, 1.5 inches long. The needle and syringe should be washed thoroughly before use. The needle and syringe should then be sterilized by placing in boiling water for 15 to 20 minutes. No vial should be entered more than 15 times with a 16 gauge needle or more than 40 times with an 18 gauge needle. The injection site should be washed with soap and water and painted with a germicide such as tincture of iodine or 70% alcohol. The product should then be administered by using the following procedure:
1. Warm the vial to room temperature and shake thoroughly to ensure uniform suspension.
2. Wipe the rubber stopper on top of the vial with a piece of absorbent cotton soaked in 70% alcohol.
3. Inject air into the vial for easier withdrawal.
4. After filling the syringe, make sure the needle is empty by pulling back the plunger of the syringe until a small air bubble appears. Then detach the needle from syringe.
5. Insert the needle deep into the muscle, attach the syringe and withdraw the plunger slightly. If blood appears, withdraw the needle and insert it into a different location.
6. Inject the dose slowly. Do not massage the site of injection.
7. Not more than 10 mL should be injected into one location.
Daily treatment should be continued for at least 48 hours after temperature has returned to normal and all other signs of infection have subsided. Animals treated with Penicillin G Procaine Injectable Suspension Alternate Method should show noticeable improvement within 36 to 48 hours.
DOSAGE: The dosage for cattle, sheep, swine, and horses is 3000 units per pound of body weight, or 1.0 mL for each 100 pounds of bodyweight, once daily. Treatment should not exceed 7 days in non-lactating dairy and beef cattle, sheep, and swine, or 5 days in lactating dairy cattle. If no improvement is observed within 48 hours, consult your veterinarian.
-
RESIDUE WARNING
RESIDUE WARNINGS:
1. Not for use in horses intended for food.
2. Milk that has been taken from animals during treatment and for 48 hours after the last treatment must not be used for food. The daily treatment schedule should not exceed seven (7) days of treatment in non-lactating dairy and beef cattle, sheep and swine, or five (5) days in lactating dairy cattle.
3. The drug should be discontinued for the following time periods before treated animals are slaughtered for food: Cattle - 4 days; Sheep - 8 days; Swine - 6 days; non-ruminating calves - 7 days.
-
PRECAUTIONS
PRECAUTIONS: Sensitivity reactions to penicillin and procaine, such as hives or respiratory distress, may occur in some animals. If such signs of sensitivity occur, stop medication and call your veterinarian. In some instances, particularly if respiratory distress is severe, immediate injection of epinephrine or antihistamine may be necessary. As with any antibiotic preparation, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If this condition is suspected, stop medication and consult your veterinarian.
- HOW SUPPLIED
- STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRO-PEN-G
penicillin g procaine injection, suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 61133-7005 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENICILLIN G PROCAINE (UNII: 17R794ESYN) (PENICILLIN G - UNII:Q42T66VG0C) PENICILLIN G 300000 [USP'U] in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61133-7005-3 250 mL in 1 VIAL, MULTI-DOSE 2 NDC: 61133-7005-2 100 mL in 1 VIAL, MULTI-DOSE 3 NDC: 61133-7005-1 500 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065505 08/09/2006 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Constant Irwindale 010569463 manufacture
Trademark Results [Pro-Pen-G]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRO-PEN-G 98309569 not registered Live/Pending |
Bimeda, Inc. 2023-12-12 |
 PRO-PEN-G 76509465 2816856 Live/Registered |
BIMEDA, INC. 2003-04-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.