CLINIQUE BROAD SPECTRUM SPF 30 SUNSCREEN FACE WITH SOLAR SMART- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone cream
CLINIQUE BROAD SPECTRUM SPF 30 SUNSCREEN FACE WITH SOLAR SMART by
Drug Labeling and Warnings
CLINIQUE BROAD SPECTRUM SPF 30 SUNSCREEN FACE WITH SOLAR SMART by is a Otc medication manufactured, distributed, or labeled by CLINIQUE LABORATORIES LLC, ESTEE LAUDER COMPANY, THE, ESTEE LAUDER COSMETICS, LTD, ESTEE LAUDER COSMETICS, LTD., ESTEE LAUDER N.V., LEN-RON MANUFACTURING DIVISION OF ARAMIS INC, NORTHTEC INC, PADC 1, WHITMAN LABORATORIES, LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
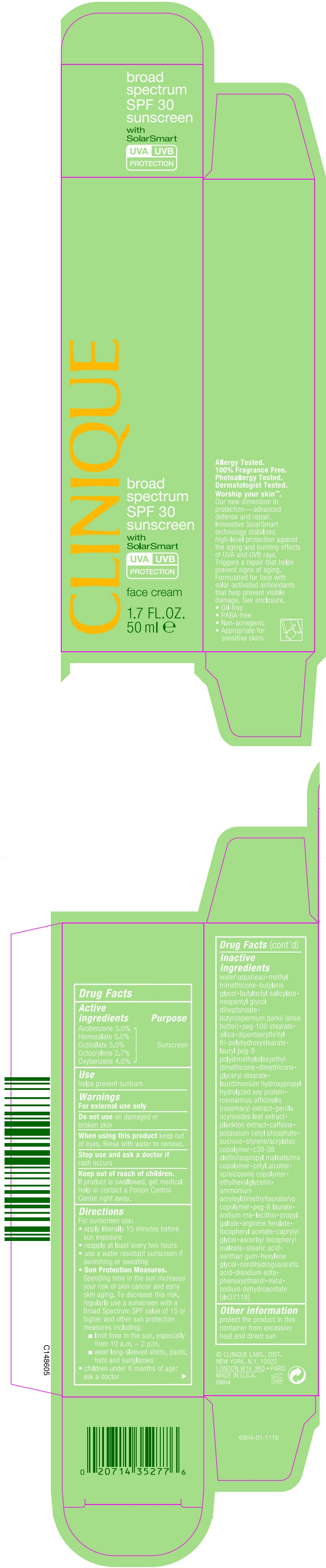
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau methyl trimethicone butylene glycol butyloctyl salicylate neopentyl glycol diheptanoate butyrospermum parkii (shea butter) peg-100 stearate silica dipentaerythrityl tri-polyhydroxystearate lauryl peg-9 polydimethylsiloxyethyl dimethicone dimethicone glyceryl stearate laurdimonium hydroxypropyl hydrolyzed soy protein rosmarinus officinalis (rosemary) extract perilla ocymoides leaf extract plankton extract caffeine potassium cetyl phosphate sucrose styrene/acrylates copolymer c30-38 olefin/isopropyl maleate/ma copolymer cetyl alcohol vp/eicosene copolymer ethylhexylglycerin ammonium acryloyldimethyltaurate/vp copolymer peg-8 laurate sodium rna lecithin propyl gallate arginine ferulate tocopheryl acetate caprylyl glycol ascorbyl tocopheryl maleate stearic acid xanthan gum hexylene glycol nordihydroguaiaretic acid disodium edta phenoxyethanol mica sodium dehydroacetate [iln37116]
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
CLINIQUE BROAD SPECTRUM SPF 30 SUNSCREEN FACE WITH SOLAR SMART
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49527-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.0309 g in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.0515 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.0515 g in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.0278 g in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.0412 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) SHEA BUTTER (UNII: K49155WL9Y) PEG-100 STEARATE (UNII: YD01N1999R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE (UNII: D21K655H52) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ROSEMARY (UNII: IJ67X351P9) CAFFEINE (UNII: 3G6A5W338E) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SUCROSE (UNII: C151H8M554) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) CETYL ALCOHOL (UNII: 936JST6JCN) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PEG-8 LAURATE (UNII: 762O8IWA10) PROPYL GALLATE (UNII: 8D4SNN7V92) ARGININE FERULATE (UNII: 0774Y45BEE) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ASCORBYL TOCOPHERYL MALEATE (UNII: D2G6259XR5) STEARIC ACID (UNII: 4ELV7Z65AP) XANTHAN GUM (UNII: TTV12P4NEE) HEXYLENE GLYCOL (UNII: KEH0A3F75J) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49527-064-01 1 in 1 CARTON 09/01/2010 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 09/01/2010 Labeler - CLINIQUE LABORATORIES LLC (044475127) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(49527-064) , REPACK(49527-064) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(49527-064) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(49527-064) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(49527-064) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(49527-064) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(49527-064) , REPACK(49527-064) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(49527-064) , REPACK(49527-064) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(49527-064) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(49527-064)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.