IMOGAM RABIES-HT- human rabies virus immune globulin injection, solution
IMOGAM RABIES-HT by
Drug Labeling and Warnings
IMOGAM RABIES-HT by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc., Sanofi Pasteur SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Rabies Immune Globulin (Human) USP, Heat Treated, Imogam® Rabies – HT, is a sterile solution of antirabies immunoglobulin (10-16% protein) for wound infiltration and intramuscular administration. Rabies immune globulin (RIG) is prepared by cold alcohol fractionation from pooled venous plasma of individuals immunized with Rabies Vaccine prepared from human diploid cells (HDCV). The product is stabilized with 0.3 M glycine. The immune globulin solution has a pH of 6.8 ± 0.4 adjusted with sodium hydroxide or hydrochloric acid. No preservatives are added. Imogam Rabies – HT is a clear or slightly opalescent, colorless or pale-yellow or light-brown liquid. During storage it may show formation of slight turbidity or a small amount of visible particulate matter.
A heat-treatment process step (58° to 60°C, 10 hours) to inactivate viruses is used to further reduce any risk of blood-borne viral transmission. The inactivation and removal of model laboratory strains of enveloped and non-enveloped viruses during the manufacturing and heat treatment processes for Imogam Rabies – HT have been validated by spiking experiments.
Human immunodeficiency virus, type 1 (HIV-1) and type 2 (HIV-2) were selected as relevant viruses for plasma derived products. Bovine viral diarrhea virus and Sindbis virus were chosen to model hepatitis C virus. Porcine pseudorabies virus was selected to model hepatitis B virus and herpes virus. Avian reovirus was used to model non-enveloped RNA viruses and for its relative resistance to inactivation by chemical and physical methods. Finally, porcine parvovirus was selected to model human parvovirus B19 and its notable resistance to inactivation by heat treatment.
Removal and/or inactivation of the spiked model viruses was demonstrated at the precipitation III stage of manufacturing. In addition, inactivation was demonstrated to occur during the 10-hour (58° to 60°C) heat treatment process for the representative enveloped and non-enveloped viruses.
The product is standardized against the United States (US) Standard Rabies Immune Globulin. The US unit of potency is equivalent to the International Unit (IU) for rabies antibody. The minimal potency is 150 IU/mL.
The vial stopper is not made with natural rubber latex.
-
CLINICAL PHARMACOLOGY
Rabies is a viral infection transmitted in the saliva of infected mammals. Worldwide, both dog and bat saliva exposures appear to be major contributors (see below) with or without apparent bites. The virus enters the central nervous system of the host causing an encephalomyelitis that is fatal.
Unlike the situation in developing countries, wild animals are the most important potential source of infection for humans and domestic animals in the US. Most reported cases of rabies occur among carnivores, primarily raccoons, skunks, and foxes and various species of bats. For the past several decades, the majority of naturally acquired, indigenous human rabies cases in the US have resulted from variants of rabies viruses associated with insectivorous bats. (1) Hawaii historically has never had an indigenous case of rabies. (2)
The United States has experienced a substantial decrease in human rabies cases acquired from indigenous domestic animals. International travelers to areas where canine rabies remains enzootic are at risk for exposure to rabies from domestic and feral dogs. (1) Infectious sources of cases of rabies in dogs in the United States in recent years were wildlife reservoirs or dogs that were translocated from localities where canine rabies virus variants still circulate.
-
INDICATIONS AND USAGE
Rabies Immune Globulin (Human) Heat Treated, Imogam Rabies – HT, in conjunction with the standard series of Rabies Vaccine vaccinations, is indicated for individuals suspected of exposure to rabies, particularly severe exposure, with one exception: persons who have been previously immunized with Rabies Vaccine. Previously immunized persons are those who have had a documented rabies virus neutralizing antibody titer and who have completed one of the recommended regimens (pre-exposure or post-exposure) with a cell culture vaccine or another vaccine. Administer only vaccine to these persons (i.e., post-exposure for a person previously vaccinated). (1)
Inject Imogam Rabies – HT, as promptly as possible after exposure, along with the first dose of vaccine. If initiation of treatment is delayed for any reason, still administer Imogam Rabies – HT and the first dose of vaccine, regardless of the interval between exposure and treatment. If Rabies Immune Globulin (Human) was not administered when vaccination was begun (i.e., day 0), it can be administered up to and including day 7 of the post-exposure prophylaxis series. (1)
In order to manage potential human exposures to rabies appropriately, the risk of infection must be accurately assessed. Rabies virus is usually transmitted by the bite of a rabid animal (dog, bat, etc.) but can occasionally penetrate abraded skin contaminated with the saliva of infected animals. Progress of the virus after exposure is believed to follow a neural pathway, and the time between exposure and clinical rabies is a function of the proximity of the bite (or abrasion) to the central nervous system and the dose of virus injected. The incubation is usually 2 to 6 weeks but can be longer. After severe bites about the face, neck, and arms, it may be as short as 10 days. After initiation of the vaccine series (human diploid cell origin), it takes approximately one week for development of immunity to rabies; therefore, the value of immediate passive immunization with rabies antibodies in the form of Rabies Immune Globulin (Human) cannot be overemphasized.
Recommendations for passive and active immunization after exposure to an animal suspected of having rabies have been outlined by the WHO (3) and by the United States Public Health Service Advisory Committee on Immunization Practices (ACIP). (1)
Centers for Disease Control (CDC) recommendations were derived from data on the safety and efficacy of active and passive rabies immunization from both uncontrolled human and animal studies.
I. Rationale of Treatment
Physicians must evaluate each possible rabies exposure. Consult local or state public health officials if questions arise about the need for treatment. Consider the following factors before specific antirabies treatment is initiated.
1. Species of Biting Animal
Bats
Rabid bats have been documented in the 49 continental states, and bats are increasingly implicated as important wildlife reservoirs for variants of rabies virus transmitted to humans. Transmission of rabies virus can occur from minor, seemingly underappreciated or unrecognized bites from bats (see Table 1). (1)
Because some bat bites may be less severe, and therefore more difficult to recognize, rabies post-exposure treatment should be considered for any physical contact with bats when bite, scratch, or mucous membrane contact cannot be excluded. (1)
Wild Terrestrial Carnivores
Raccoons, skunks, and foxes are the terrestrial carnivores most often infected with rabies in the United States. Suggestive clinical signs of rabies among wildlife cannot be interpreted reliably. All bites by such wildlife should be considered possible exposures to rabies virus. Post-exposure prophylaxis should be initiated as soon as possible following suspected rabies virus exposure to such wildlife, unless the animal is available for diagnosis and public health authorities are facilitating expeditious laboratory testing, or if the brain tissue from the animal has already tested negative (see Table 1). (1)
Other Wild Animals
Small rodents (e.g., squirrels, chipmunks, rats, mice, hamsters, guinea pigs, and gerbils) and lagomorphs (including rabbits and hares) are rarely infected with rabies and have not been known to transmit rabies to humans. In all cases involving rodents, the state or local health department should be consulted before a decision is made to initiate post-exposure prophylaxis (see Table 1). (1)
Domestic Dogs, Cats, and Ferrets
The likelihood of rabies in a domestic animal varies regionally, and the need for post-exposure prophylaxis also varies on the basis of regional epidemiology (see Table 1). (1)
2. Circumstances of Biting Incident
An unprovoked attack might be more likely than a provoked attack to indicate the animal is rabid. Bites inflicted on a person attempting to feed or handle an apparently healthy animal should generally be regarded as provoked. Consult the local or state health department following a provoked or unprovoked exposure to determine the best course of action based on current public health recommendations.
3. Type of Exposure
Rabies is commonly transmitted by inoculation with infectious saliva. The likelihood that rabies infection will result from exposure to a rabid animal varies with the nature and extent of the exposure. Two categories of exposure should be considered, bite and nonbite.
Nonbite
Scratches, abrasions, open wounds or mucous membranes contaminated with saliva or other potentially infectious material such as brain tissue from a rabid animal. Indirect contact and activities (e.g., petting or handling an animal, contact with blood, urine or feces, and contact of saliva with intact skin) do not constitute exposures. Rare reports of aerosol exposure have been received from laboratory and bat-infested cave settings. (1)
Rare cases of rabies from human-to-human transmission have occurred in patients in the US and overseas who received organs transplanted from persons who died of rabies undiagnosed at the time of death. No documented laboratory-diagnosed cases of human-to-human rabies transmission have been documented from a bite or nonbite exposure other than the transplant cases. At least two cases of human-to-human rabies transmission in Ethiopia have been suggested, but rabies as the cause of death was not confirmed by laboratory testing. The reported route of exposure in both cases was direct salivary contact from another human (i.e., a bite and a kiss). Routine delivery of health care to a patient with rabies is not an indication for post-exposure prophylaxis unless the healthcare worker is reasonably certain that he or she was bitten by the patient or that his or her mucous membranes or nonintact skin was exposed directly to potentially infectious saliva or neural tissue. (1)
II. Post-exposure Treatment of Rabies
The essential components of rabies post-exposure prophylaxis are wound treatment and, for previously unvaccinated persons, the administration of both human rabies immune globulin (RIG) and vaccine. (1)
1. Local Treatment of Wounds
Thoroughly wash and flush all bite wounds and scratches immediately or as early as possible (for about 15 minutes, if possible) with soap or a cleansing agent and copious amounts of water. Where available, apply an iodine-containing, or similarly viricidal, topical preparation to the wound. (3)
Administer Tetanus prophylaxis and measures to control bacterial infection, as indicated.
2. Specific Treatment
Administer post-exposure antirabies vaccination with rabies vaccine in addition to administering Rabies Immune Globulin (RIG). However, for persons who have previously received complete vaccination regimens (pre-exposure or post-exposure) with a cell culture vaccine or persons who have been vaccinated with other types of vaccines and have had documented rabies antibody titers, administer the vaccine alone. The combination (administered at different sites) of Rabies Immune Globulin (RIG) and vaccine is recommended for both bite exposures and nonbite exposures (see Rationale of Treatment), regardless of the interval between exposure and initiation of treatment. (1)
3. Post-exposure Treatment Guide
The following recommendations are only a guide. Apply these in conjunction with knowledge of the animal species involved, circumstances of the bite or other exposure, vaccination status of the animal, and presence of rabies in the region. Consult local and state public health officials if questions arise about the need for rabies prophylaxis.
Table 1: RABIES POST-EXPOSURE PROPHYLAXIS GUIDE, UNITED STATES (1) Animal Type Evaluation and Disposition of Animal Post-exposure Prophylaxis Recommendations - * During the 10-day observation period, begin post-exposure prophylaxis at the first sign of rabies in a dog, cat, or ferret that has bitten someone. If the animal exhibits clinical signs of rabies, it should be euthanized immediately and tested.
- † Initiate post-exposure prophylaxis as soon as possible following exposure to such wildlife unless the animal is available for testing and public health authorities are facilitating expeditous laboratory testing or it is already known that brain material from the animal has tested negative. Other factors that might influence the urgency of decision-making regarding initiation of post-exposure prophylaxis before diagnostic results are known include the species of the animal, the general appearance and behavior of the animal, whether the encounter was provoked by the presence of a human, and the severity and location of bites. Discontinue vaccine if appropriate laboratory diagnostic test (i.e., the direct fluorescent antibody test) is negative.
- ‡ The animal should be euthanized and tested as soon as possible. Holding for observation is not recommended.
Dogs, cats, and ferrets Healthy and available for 10 days observation Persons should not begin prophylaxis unless animal develops clinical signs of rabies.* Rabid or suspected rabid Immediately begin prophylaxis. Unknown (e.g., escaped) Consult public health officials. Wild skunks, raccoons, foxes, and most other carnivores; bats† Regarded as rabid unless animal proven negative by laboratory tests‡ Consider immediate prophylaxis. Livestock, small rodents, lagomorphs (rabbits and hares), large rodents (woodchucks and beavers), and other mammals Consider individually Consult public health officials. Bites from squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice, other small rodents, rabbits, and hares almost never require antirabies post-exposure prophylaxis. - CONTRAINDICATIONS
-
WARNINGS
- Administration of rabies post-exposure prophylaxis is a medical urgency, not a medical emergency, but decisions must not be delayed.
- Although no post-exposure vaccine failures have occurred in the US since cell culture vaccines have been routinely used, failures have occurred abroad when some deviation was made from the recommended post-exposure treatment protocol or when less than the currently recommended amount of antirabies sera was administered. (1)
- Rabies Immune Globulin (Human) USP, Heat Treated, Imogam Rabies – HT, is made from human plasma. Because this product is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. An alcohol fractionation procedure used to purify the immunoglobulin component removes and/or inactivates both enveloped and non-enveloped viruses to a certain extent. An added heat treatment process (60°C, 10 hours) further inactivates both enveloped and non-enveloped viruses. Despite these measures, it is still theoretically possible that known or unknown infectious agents may be present. Report all infections thought by a physician possibly to have been transmitted by this product to the Pharmacovigilance Department, Sanofi Pasteur Inc., 1-800-822-2463. Discuss the risks and benefits of this product with the patient.
- Prior to administration, review the patient history for possible sensitivity to human immune globulin. Epinephrine injection (1:1000) must be immediately available should an acute anaphylactic reaction occur.
- Persons with IgA deficiency have increased potential for developing antibodies to IgA and could have anaphylactic reactions to subsequent administration of blood products containing IgA. (4) (5)
-
PRECAUTIONS
- Imogam Rabies – HT is not for intravenous administration. Do NOT administer intravenously.
- Never administer Rabies Immune Globulin (Human) in the same syringe or into the same anatomical site as the vaccine dose. Because Rabies Immune Globulin (Human) may partially suppress active production of antibody, no more than the recommended dose should be given. (1)
- Use a separate, sterile syringe and needle or a sterile disposable unit for each patient to prevent transmission of hepatitis or other infectious agents from person to person. Do not recap needles. Dispose of needles and syringes according to local biohazard waste guidelines.
- INFORMATION FOR VACCINE RECIPIENTS OR PARENTS/GUARDIANS
- DRUG INTERACTIONS
-
PREGNANCY
Animal reproduction studies have not been conducted with Imogam Rabies – HT. It is also not known whether Imogam Rabies – HT can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Administer Imogam Rabies – HT to a pregnant woman only if clearly needed.
Because of the potential consequences of inadequately treated rabies exposure and limited data that indicate that fetal abnormalities have not been associated with rabies vaccination, pregnancy is not considered a contraindication to post-exposure prophylaxis. If there is substantial risk of exposure to rabies, pre-exposure prophylaxis may also be indicated during pregnancy. (1)
- LACTATION
-
ADVERSE REACTIONS
Systemic prophylactic treatments occasionally are complicated by adverse reaction. (1)
Data From Clinical Studies
The safety profile of Imogam Rabies – HT (heat treated) was compared to Rabies Immune Globulin (Human), Imogam Rabies (non-heat treated), in a clinical trial involving 16 volunteers in each of 4 treatment groups (64 total subjects). Local and systemic adverse reactions were classified by the description of the reaction and by its severity1. Both Imogam Rabies – HT and Imogam Rabies were without reported serious adverse reactions or allergic reactions. Two subjects reported severe headaches, one in the Imogam Rabies – HT + placebo group and one in the Imogam Rabies + Imovax® Rabies group, and one third of the volunteers reported moderate systemic (headache and malaise) reactions. These were equally distributed among the 4 treatment groups with no significant differences between the groups. Three-quarters of the local adverse reactions (tenderness, pain, erythema, induration, pruritus, regional adenopathy) were mild. The safety profile did not differ between groups, although Imogam Rabies – HT produced fewer and milder local reactions such as pain or tenderness at the injection site. (6)
- 1 Mild - aware of symptoms but easily tolerated [pain described as tenderness upon touch; erythema, bruising, induration/ swelling <1 inch (2.5 cm); occasional site pruritis; fever (oral temperature 37.5-38.5°C)]. Moderate - discomforting enough to interfere with normal daily activity [pain on movement; erythema, bruising, induration/swelling of 1–2 inches (2.5–5 cm) in diameter; frequent site pruritis; fever (oral temperature 38.5-40°C)]. Severe - subject bed-ridden [disabling pain that limited motion; erythema, bruising, induration/ swelling >2 inches (>5 cm); continuous site pruritis, fever (oral temperature >40°C).
Data From Post-marketing Experience
The following additional adverse reactions have been identified during postapproval use of Imogam Rabies – HT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Imogam Rabies – HT exposure.
Cardiac Disorders
- Hypotension, tachycardia
Gastrointestinal Disorders
- Nausea, vomiting
General Disorders and Administration Site Conditions
- Fever, chills
Immune System Disorders
- Anaphylaxis, allergic reaction
Skin and Subcutaneous System Disorders
- Rash, pruritus
Data From Literature
Although not reported specifically for Rabies Immune Globulin (Human), angioneurotic edema and nephrotic syndrome have been reported after injection of immune globulin (IG), a product similar in biochemical composition but without antirabies activity. (7)
Reporting of Adverse Reactions
Encourage reporting by patients, parents or guardians of all suspected adverse reactions occurring after Rabies Immune Globulin (Human) administration. Report suspected adverse reactions following treatment with Rabies Immune Globulin (Human) to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Also report these events to the Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 or call 1-800-822-2463.
-
DOSAGE AND ADMINISTRATION
For wound infiltration and intramuscular administration only.
Inspect parenteral drug products visually for particulate matter and/or discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, do not administer the product.
Use Imogam Rabies – HT in conjunction with rabies vaccines such as Rabies Vaccine, Imovax Rabies, for intramuscular immunization, a vaccine prepared from human diploid cell cultures. The recommended dose of Imogam Rabies – HT is 20 IU/kg (0.133 mL/kg) or 9 IU/lb (0.06 mL/lb) of body weight administered at the time of the first vaccine dose. (3) (8) (9)
If anatomically feasible, use the full dose of Rabies Immune Globulin (Human) to thoroughly infiltrate the area around and into the wounds. Inject any remaining volume intramuscularly, using a separate needle, at a site distant from vaccine administration. (1) (10)
Never administer Rabies Immune Globulin (Human) in the same syringe or into the same anatomical site as rabies vaccine. Because Rabies Immune Globulin (Human) may partially suppress active production of antibody, do not give more than the recommended dose. (1) (11)
-
HOW SUPPLIED
Imogam Rabies – HT is supplied in a 2 mL vial with a minimal potency of 150 International Units per milliliter (IU/mL).
Vial, 2 mL, NDC: 49281-190-58. Packaged as NDC: 49281-190-20.
Imogam Rabies – HT contains no preservative. Discard unused portion immediately.
- STORAGE
-
CLINICAL STUDIES
Controlled human trials of Rabies Immune Globulin (Human) have not been performed; however, extensive field experience from many areas of the world indicates that post-exposure prophylaxis combining local wound treatment, local infiltration of rabies immune globulin (RIG), and vaccination is uniformly effective when appropriately administered. (1)
Rabies antibody provides passive protection when given immediately to individuals exposed to rabies virus. (12) Studies of Rabies Immune Globulin (Human), (11) Imogam Rabies, given with the first of five doses of Sanofi Pasteur SA HDCV (7) confirmed that passive immunization with 20 IU/kg of Rabies Immune Globulin (Human) provides maximum circulating antibody with minimum interference of active immunization by HDCV.
A double-blind randomized trial (6) was conducted to compare the safety and antibody levels achieved following intramuscular injection of Imogam Rabies – HT (heat treated) and Rabies Immune Globulin (Human), Imogam Rabies (non-heat treated). Each Rabies Immune Globulin (Human) was administered on day 0, either alone or in combination with the human diploid cell Rabies Vaccine (Imovax Rabies) using the standard post-exposure prophylactic schedule of day 0, 3, 7, 14, and 28.
Sixty-four healthy veterinary student volunteers were randomized into four parallel groups of 16 each to receive the following Rabies Immune Globulin (Human) and vaccine regimens:
- Imogam Rabies – HT + Imovax
- Imogam Rabies + Imovax
- Imogam Rabies – HT + placebo
- Imogam Rabies + placebo
The treatment of both Rabies Immune Globulin (Human) and vaccine corresponded to the post-exposure recommended dose of 20 IU/kg of Rabies Immune Globulin (Human) and was administered in three equally divided IM injections of under 5 mL in either gluteus. Serum rabies antibody levels were assessed before treatment and on days 3, 7, 14, 28, 35, and 42 by the Rabies Fluorescent Focus Inhibition Test (RFFIT).
Serum antibody levels were similar in the Imogam Rabies – HT and Imogam Rabies groups. By day three, 60% of each group had detectable antibody titers of ≥0.05 IU/mL. By day 14, the geometric mean titers (with 95% confidence interval) were 19 IU/mL (11-38) in the Imogam Rabies – HT + vaccine group and 31 IU/mL (20 to 48) in the Imogam Rabies + vaccine group. These differences were not statistically significant.
-
REFERENCES
- 1 Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, et al. Human rabies prevention - United States 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2008 May 23;57(RR-3):1-28.
- 2 State of Hawaii Animal Industry Division. Available at: http://hdoa.hawaii.gov/ai/aqs/. Accessed January 21, 2014.
- 3 Rabies vaccines: WHO position paper. Weekly epidemiological record. 2010 Aug 6;85(32): 309-320. Available from http://www.who.int/wer. Accessed January 21, 2014.
- 4 Fudenberg HH. Sensitization to immunoglobulins and hazards of gamma globulin therapy, pp 211-220 in Merler E, Editor Immunoglobulins: biologic aspects and clinical uses. National Academy of Sciences, Wash., DC. 1970.
- 5 Pineda AA, et al. Transfusion reactions associated with anti-IgA antibodies: report of four cases and review of the literature. Transfusion 1975;15:10-15.
- 6 Lang J, et al. Evaluation of the safety and immunogenicity of a new, heat-treated human rabies immune globulin using a sham, post-exposure prophylaxis of rabies. Biologicals 1998;26:7-15.
- 7 Recommendation of the Advisory Committee on Immunization Practices (ACIP). Human Rabies Prevention - United States 1999. MMWR 1999;48:No. RR-1.
- 8 Cabasso VJ, et al. Rabies immune globulin of human origin: preparation and dosage determination in non-exposed volunteer subjects. Bull WHO 1971;45:303-315.
- 9 Loofbourow JC, et al. Rabies immune globulin (human). Clinical trials and dose determination. JAMA 1971;217:1825-1831.
- 10 Fishbein DB, et al. Administration of human diploid-cell rabies vaccine in the gluteal area. N Engl J Med 1988;318:124-125.
- 11 Helmick CG, et al. A clinical study of Mérieux human rabies immune globulin. J Biol Stand 1982;10:357-367.
- 12 Habel K, et al. Laboratory data supporting clinical trial of antirabies serum in persons bitten by rabid wolf. Bull WHO 1955;13:773-779.
- SPL UNCLASSIFIED SECTION
-
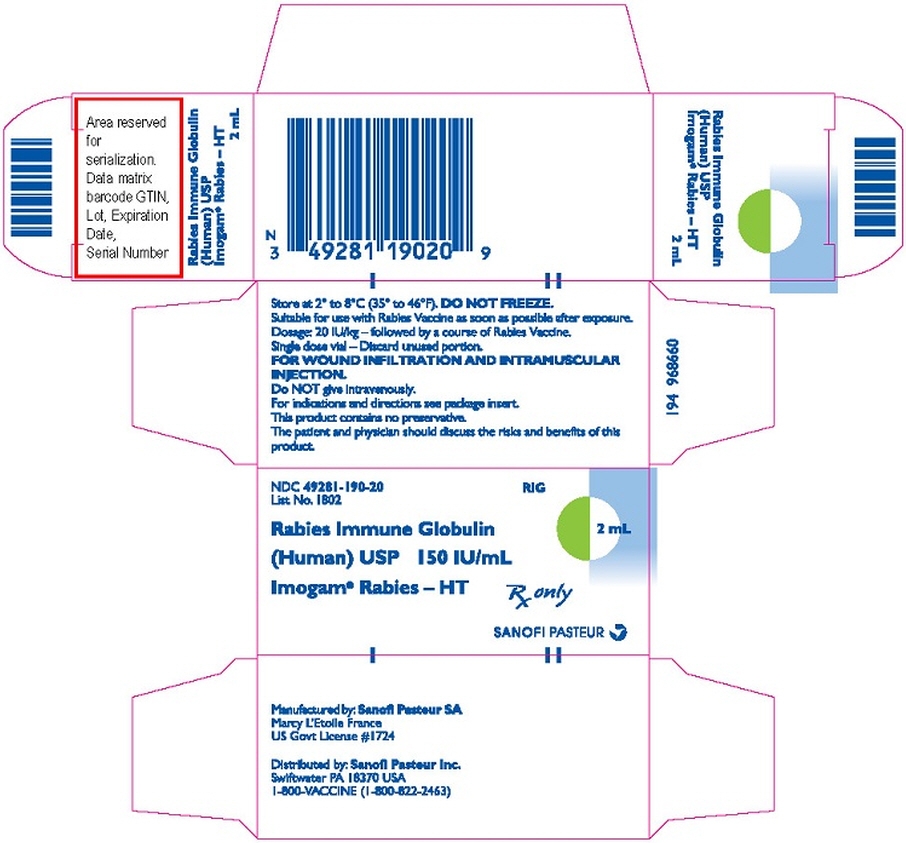
PRINCIPAL DISPLAY PANEL - 2 mL Vial Label
Rabies Immune
Globulin (Human)
USP 150 IU/mL
RIG
Imogam® Rabies – HT2 mL
Dosage: see insert. The patient
and physician should discuss the
risks and benefits of this product.Mfd. by: Sanofi Pasteur SA
Marcy L'Etoile France US Govt Lic #1724Dist. by: Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
1-800-VACCINE (1-800-822-2463)Rx only

-
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
NDC: 49281-190-20
List No. 1802RIG
2 mL
Rabies Immune Globulin
(Human) USP 150 IU/mL
Imogam® Rabies – HTRx only
SANOFI PASTEUR
-
INGREDIENTS AND APPEARANCE
IMOGAM RABIES-HT
human rabies virus immune globulin injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 49281-190 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN RABIES VIRUS IMMUNE GLOBULIN (UNII: 95F619ATQ2) (HUMAN RABIES VIRUS IMMUNE GLOBULIN - UNII:95F619ATQ2) HUMAN RABIES VIRUS IMMUNE GLOBULIN 150 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) 22.5 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 1 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-190-20 1 in 1 CARTON 1 NDC: 49281-190-58 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103932 04/27/1984 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur SA 578763542 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.