TASCENSO ODT- fingolimod lauryl sulfate tablet, orally disintegrating
TASCENSO ODT by
Drug Labeling and Warnings
TASCENSO ODT by is a Prescription medication manufactured, distributed, or labeled by Cycle Pharmaceuticals Ltd, Catalent Pharma Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TASCENSO ODT® safely and effectively. See full prescribing information for TASCENSO ODT.
TASCENSO ODT (fingolimod) orally disintegrating tablets
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
INDICATIONS AND USAGE
TASCENSO ODT is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- Assessments are required prior to initiating TASCENSO ODT (2.1)
- Recommended dosage for adults and pediatric patients (10 years of age and older) weighing more than 40 kg: 0.5 mg orally once daily, with or without food. (2.2, 2.3)
- Recommended dosage for pediatric patients (10 years of age and older) weighing less than or equal to 40 kg: 0.25 mg orally once daily, with or without food (2.2, 2.3).
- Administer TASCENSO ODT with or without water. Place tablet directly on the tongue and allow it to dissolve before swallowing. (2.2)
- First-Dose Monitoring (including reinitiation after discontinuation greater than 14 days and dose increases):
- Observe all patients for bradycardia for at least 6 hours; monitor pulse and blood pressure hourly. Electrocardiograms (ECGs) prior to dosing and at end of observation period required. (2.4)
- Monitor until resolution if heart rate < 45 beats per minute (bpm) in adults, < 55 bpm in patients aged 12 years and above, or < 60 bpm in pediatric patients aged 10 to below 12 years, atrioventricular (AV) block, or if lowest postdose heart rate is at the end of the observation period. (2.4)
- Monitor symptomatic bradycardia with ECG until resolved. Continue overnight if intervention is required; repeat first-dose monitoring for second dose. (2.4)
- Observe patients overnight if at higher risk of symptomatic bradycardia, heart block, prolonged QTc interval, or if taking drugs with known risk of torsades de pointes. (2.4, 7.1)
DOSAGE FORMS AND STRENGTHS
Orally disintegrating tablets: 0.25 mg and 0.5 mg (3)
CONTRAINDICATIONS
- Recent myocardial infarction, unstable angina, stroke, transient ischemic attack, decompensated heart failure with hospitalization, or Class III/IV heart failure. (4)
- History of Mobitz Type II 2nd degree or 3rd degree AV block or sick sinus syndrome, unless patient has a pacemaker. (4)
- Baseline QTc interval ≥ 500 msec. (4)
- Cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs. (4)
- Hypersensitivity to fingolimod or its excipients. (4)
- Concomitant use with other products containing fingolimod. (4)
WARNINGS AND PRECAUTIONS
- Bradyarrhythmia and Atrioventricular Blocks: Because of a risk for bradyarrhythmia and AV blocks, monitor during initiation of treatment (2.4, 5.1)
- Infections: TASCENSO ODT may increase the risk. Obtain a complete blood count (CBC) before initiating TASCENSO ODT (i.e., within 6 months). Monitor for infection during treatment and for 2 months after discontinuation. Do not start in patients with active infections. (5.2)
- Progressive Multifocal Leukoencephalopathy (PML): Withhold TASCENSO ODT at the first sign or symptom suggestive of PML. (5.3)
- Macular Edema: Increases the risk of macular edema. Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with TASCENSO ODT. Conduct an evaluation of the fundus, including the macula, 3 to 4 months after treatment start, periodically while on therapy, and any time there is a change in vision. Consider discontinuing TASCENSO ODT if macular edema develops. Diabetes mellitus and uveitis increase the risk. (5.4)
- Liver Injury: Obtain liver enzyme results before initiation and periodically during treatment. Closely monitor patients with severe hepatic impairment. Discontinue if there is evidence of liver injury without other cause. (5.5, 8.6, 12.3)
- Posterior Reversible Encephalopathy Syndrome (PRES): If suspected, discontinue TASCENSO ODT. (5.6)
- Respiratory Effects: Evaluate when clinically indicated. (5.7)
- Fetal Risk: May cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during treatment and for 2 months after stopping TASCENSO ODT. (5.8, 8.1, 8.3)
- Severe Increase in Disability After Stopping TASCENSO ODT: Monitor for development of severe increase in disability following discontinuation and begin appropriate treatment as needed. (5.9)
- Tumefactive MS: Consider when severe MS relapse occurs during treatment or after discontinuation. Obtain imaging and begin treatment as needed. (5.10)
- Increased Blood Pressure (BP): Monitor BP during treatment. (5.11)
- Malignancies: Skin examination prior to or shortly after the start of treatment and periodically thereafter is recommended. Suspicious skin lesions should be evaluated. (5.12)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 10% and greater than placebo): Headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Cycle Pharmaceuticals Ltd at 1-888-533-1625 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Assessment Prior to Initiating TASCENSO ODT
2.2 Important Administration Instructions
2.3 Recommended Dosage
2.4 First-Dose Monitoring
2.5 Monitoring After Reinitiation of Therapy Following Discontinuation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bradyarrhythmia and Atrioventricular Blocks
5.2 Infections
5.3 Progressive Multifocal Leukoencephalopathy
5.4 Macular Edema
5.5 Liver Injury
5.6 Posterior Reversible Encephalopathy Syndrome
5.7 Respiratory Effects
5.8 Fetal Risk
5.9 Severe Increase in Disability After Stopping TASCENSO ODT
5.10 Tumefactive Multiple Sclerosis
5.11 Increased Blood Pressure
5.12 Malignancies
5.13 Immune System Effects Following TASCENSO ODT Discontinuation
5.14 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 QT Prolonging Drugs
7.2 Ketoconazole
7.3 Vaccines
7.4 Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies
7.5 Drugs That Slow Heart Rate or Atrioventricular Conduction (e.g., beta blockers or diltiazem)
7.6 Laboratory Test Interaction
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adults
14.2 Pediatric Patients (10 to less than 18 Years of Age)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Assessment Prior to Initiating TASCENSO ODT
Cardiac Evaluation
Obtain a cardiac evaluation in patients with certain preexisting conditions [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
Prior to starting treatment, determine whether patients are taking drugs that could slow heart rate or atrioventricular (AV) conduction [see Dosage and Administration (2.4), Drug Interactions (7.5)].
Complete Blood Count (CBC)
Review results of a recent CBC [see Warnings and Precautions (5.2), Drug Interactions (7.6)].
Serum Transaminases (ALT and AST) and Total Bilirubin Levels
Prior to starting treatment with TASCENSO ODT (i.e., within 6 months), review serum transaminases [alanine transaminase (ALT) and aspartate transferase (AST)] and total bilirubin levels [see Warnings and Precautions (5.5)].
Ophthalmic Assessment
Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with TASCENSO ODT [see Warnings and Precautions (5.4)].
Skin Examination
Obtain a baseline skin examination prior to or shortly after initiation of TASCENSO ODT. If a suspicious skin lesion is observed, it should be promptly evaluated [see Warnings and Precautions (5.12)].
Prior Medications
If patients are taking antineoplastic, immunosuppressive, or immune-modulating therapies, or if there is a history of prior use of these drugs, consider possible unintended additive immunosuppressive effects prior to initiating treatment with TASCENSO ODT [see Warnings and Precautions (5.2), Drug Interactions (7.4)].
Vaccinations
Prior to initiating TASCENSO ODT, test patients for antibodies to varicella zoster virus (VZV). VZV vaccination of antibody- negative patients is recommended prior to commencing treatment with TASCENSO ODT [see Warnings and Precautions (5.2)]. It is recommended that pediatric patients if possible, complete all other immunizations in accordance with current immunization guidelines prior to initiating TASCENSO ODT.
2.2 Important Administration Instructions
Patients who initiate TASCENSO ODT, and those who reinitiate treatment after discontinuation for longer than 14 days, require first- dose monitoring. This monitoring is also recommended when the dose is increased in pediatric patients. Patients who are currently treated with another fingolimod product and underwent first-dose monitoring at initiation may be switched to TASCENSO ODT at the same daily dose without a need to repeat first-dose monitoring (unless the previous treatment was discontinued more than 14 days prior) [see Dosage and Administration (2.4, 2.5)].
TASCENSO ODT can be taken with or without food.
Inform the patient about the following administration instructions for TASCENSO ODT:
- Store the orally disintegrating tablet (ODT) in the sealed blister until ready to administer.
- Do not push the ODT through the lidding foil.
- Use dry hands when opening the blister pack.
- Peel back the lidding foil from one blister, then push the underside of the foil packet to release the ODT and gently remove the ODT.
- Take the ODT immediately after opening the blister pack.
- Place the ODT on the tongue and allow it to dissolve before swallowing.
- The ODT may be taken with or without water.
2.3 Recommended Dosage
In adults and pediatric patients 10 years of age and older weighing more than 40 kg, the recommended dosage of TASCENSO ODT is 0.5 mg orally once-daily.
In pediatric patients 10 years of age and older weighing less than or equal to 40 kg, the recommended dosage of TASCENSO ODT is 0.25 mg orally once daily.
Fingolimod doses higher than 0.5 mg are associated with a greater incidence of adverse reactions without additional benefit.
2.4 First-Dose Monitoring
Initiation of TASCENSO ODT treatment results in a decrease in heart rate, for which monitoring is recommended [see Warnings and Precautions (5.1), Clinical Pharmacology (12.2)]. Prior to dosing and at the end of the observation period, obtain an electrocardiogram (ECG) in all patients.
First 6-Hour Monitoring
Administer the first dose of TASCENSO ODT in a setting in which resources to appropriately manage symptomatic bradycardia are available. Monitor all patients for 6 hours after the first dose for signs and symptoms of bradycardia with hourly pulse and blood pressure measurement.
Additional Monitoring after 6-Hour Monitoring
Continue monitoring until the abnormality resolves if any of the following is present (even in the absence of symptoms) after 6 hours:
- The heart rate 6 hours postdose is less than 45 bpm in adults, less than 55 bpm in pediatric patients 12 years of age and older, or less than 60 bpm in pediatric patients 10 or 11 years of age
- The heart rate 6 hours postdose is at the lowest value postdose suggesting that the maximum pharmacodynamic effect on the heart may not have occurred
- The ECG 6 hours postdose shows new onset second degree or higher AV block.
If postdose symptomatic bradycardia occurs, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 6-hour monitoring after the second dose.
Overnight Monitoring
Continuous overnight ECG monitoring in a medical facility should be instituted:
- in patients that require pharmacologic intervention for symptomatic bradycardia. In these patients, the first-dose monitoring strategy should be repeated after the second dose of TASCENSO ODT
- in patients with some preexisting heart and cerebrovascular conditions [see Warnings and Precautions (5.1)]
- in patients with a prolonged QTc interval before dosing or during 6-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes [see Warnings and Precautions (5.1), Drug Interactions (7.1)]
- in patients receiving concurrent therapy with drugs that slow heart rate or AV conduction [see Drug Interactions (7.5)].
2.5 Monitoring After Reinitiation of Therapy Following Discontinuation
When restarting TASCENSO ODT treatment after discontinuation for more than 14 days after the first month of treatment, perform first-dose monitoring, because effects on heart rate and AV conduction may recur on reintroduction of TASCENSO ODT treatment [see Dosage and Administration (2.4)]. The same precautions (first-dose monitoring) as for initial dosing are applicable. Within the first 2 weeks of treatment, first-dose procedures are recommended after interruption of 1 day or more; during Weeks 3 and 4 of treatment, first-dose procedures are recommended after treatment interruption of more than 7 days.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TASCENSO ODT is contraindicated in patients who have:
- in the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure
- a history or presence of Mobitz Type II second-degree or third-degree AV block or sick sinus syndrome, unless patient has a functioning pacemaker [see Warnings and Precautions (5.1)]
- a baseline QTc interval ≥ 500 msec
- cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs
- had a hypersensitivity reaction to fingolimod or any of the excipients in TASCENSO ODT. Observed reactions include rash, urticaria, and angioedema [see Warnings and Precautions (5.14)].
- Concomitant use with other products containing fingolimod
-
5 WARNINGS AND PRECAUTIONS
5.1 Bradyarrhythmia and Atrioventricular Blocks
Because of a risk for bradyarrhythmia and AV blocks, patients should be monitored during TASCENSO ODT treatment initiation [see Dosage and Administration (2.4)].
Reduction in Heart Rate
After the first dose of TASCENSO ODT, the heart rate decrease starts within an hour. On Day 1, the maximum decline in heart rate generally occurs within 6 hours and recovers, although not to baseline levels, by 8 to 10 hours postdose. Because of physiological diurnal variation, there is a second period of heart rate decrease within 24 hours after the first dose. In some patients, heart rate decrease during the second period is more pronounced than the decrease observed in the first 6 hours. Heart rates below 40 beats per minute (bpm) in adults, and below 50 bpm in pediatric patients occurred rarely. In controlled clinical trials in adult patients, adverse reactions of symptomatic bradycardia following the first dose were reported in 0.6% of patients receiving fingolimod 0.5 mg and in 0.1% of patients on placebo. Patients who experienced bradycardia were generally asymptomatic, but some patients experienced hypotension, dizziness, fatigue, palpitations, and/or chest pain that usually resolved within the first 24 hours on treatment.
Patients with some preexisting conditions (e.g., ischemic heart disease, history of myocardial infarction, congestive heart failure, history of cardiac arrest, cerebrovascular disease, uncontrolled hypertension, history of symptomatic bradycardia, history of recurrent syncope, severe untreated sleep apnea, AV block, sinoatrial heart block) may poorly tolerate the TASCENSO ODT-induced bradycardia, or experience serious rhythm disturbances after the first dose of TASCENSO ODT. Prior to treatment with TASCENSO ODT, these patients should have a cardiac evaluation by a physician appropriately trained to conduct such evaluation, and if treated with TASCENSO ODT, should be monitored overnight with continuous ECG in a medical facility after the first dose.
Since initiation of TASCENSO ODT treatment results in decreased heart rate and may prolong the QT interval, patients with a prolonged QTc interval (> 450 msec adult and pediatric males, > 470 msec adult females, or > 460 msec pediatric females) before dosing or during 6-hour observation, or at additional risk for QT prolongation (e.g., hypokalemia, hypomagnesemia, congenital long- QT syndrome), or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility.
Following the second fingolimod dose, a further decrease in heart rate may occur when compared to the heart rate prior to the second dose, but this change is of a smaller magnitude than that observed following the first dose. With continued fingolimod dosing, the heart rate returns to baseline within 1 month of chronic treatment. Clinical data indicate effects of TASCENSO ODT on heart rate are maximal after the first dose although milder effects on heart rate may persist for, on average, 2 to 4 weeks after initiation of therapy at which time heart rate generally returns to baseline. Physicians should continue to be alert to patient reports of cardiac symptoms.
Atrioventricular Blocks
Initiation of TASCENSO ODT treatment has resulted in transient AV conduction delays. In controlled clinical trials in adult patients, first-degree AV block after the first dose occurred in 4.7% of patients receiving fingolimod capsules and 1.6% of patients on placebo. In a study of 697 patients with available 24-hour Holter monitoring data after their first dose (N = 351 receiving fingolimod capsules and N = 346 on placebo), second-degree AV blocks (Mobitz Types I [Wenckebach] or 2:1 AV blocks) occurred in 4% (N = 14) of patients receiving fingolimod capsules and 2% (N = 7) of patients on placebo. Of the 14 patients receiving fingolimod capsules, 7 patients had 2:1 AV block (5 patients within the first 6 hours postdose and 2 patients after 6 hours postdose). All second-degree AV blocks on placebo were Mobitz Type I and occurred after the first 12 hours postdose. The conduction abnormalities were usually transient and asymptomatic, and resolved within the first 24 hours on treatment, but they occasionally required treatment with atropine or isoproterenol.
Postmarketing Experience
In the postmarketing setting, third-degree AV block and AV block with junctional escape have been observed during the first-dose 6-hour observation period with fingolimod. Isolated delayed onset events, including transient asystole and unexplained death, have occurred within 24 hours of the first dose. These events were confounded by concomitant medications and/or preexisting disease, and the relationship to fingolimod is uncertain. Cases of syncope were also reported after the first dose of fingolimod.
5.2 Infections
Risk of Infections
TASCENSO ODT causes a dose-dependent reduction in peripheral lymphocyte count to 20%-30% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues. TASCENSO ODT may therefore increase the risk of infections, some serious in nature [see Clinical Pharmacology (12.2)]. Life-threatening and fatal infections have occurred in association with fingolimod, the active moiety in TASCENSO ODT.
Before initiating treatment with TASCENSO ODT, results from a recent CBC (i.e., within 6 months or after discontinuation of prior therapy) should be reviewed. Initiation of treatment with TASCENSO ODT should be delayed in patients with severe active infection until resolution. Because residual pharmacodynamic effects, such as lowering effects on peripheral lymphocyte count, may persist for up to 2 months after discontinuation of TASCENSO ODT, vigilance for infection should be continued throughout this period [see Warnings and Precautions (5.13)].
In MS placebo-controlled trials in adult patients treated with fingolimod capsules, the overall rate of infections (72%) with fingolimod capsules was similar to placebo. However, bronchitis, herpes zoster, influenza, sinusitis, and pneumonia were more common in fingolimod-treated patients. Serious infections occurred at a rate of 2.3% in the fingolimod group versus 1.6% in the placebo group.
In the postmarketing setting, serious infections with opportunistic pathogens including viruses (e.g., John Cunningham virus (JCV), herpes simplex viruses 1 and 2, varicella zoster virus), fungi (e.g., cryptococci), and bacteria (e.g., atypical mycobacteria) have been reported with fingolimod. Patients with symptoms and signs consistent with any of these infections should undergo prompt diagnostic evaluation and appropriate treatment.
Herpes Viral Infections
In placebo-controlled trials in adult patients treated with fingolimod capsules, the rate of herpetic infections was 9% in patients receiving fingolimod 0.5 mg and 7% on placebo.
Two patients died of herpetic infections during controlled trials. One death was due to disseminated primary herpes zoster and the other was to herpes simplex encephalitis. In both cases, the patients were taking a 1.25 mg dose of fingolimod (higher than the recommended 0.5 mg dose) and had received high-dose corticosteroid therapy to treat suspected MS relapses.
Serious, life-threatening events of disseminated varicella zoster and herpes simplex infections, including cases of encephalitis and multiorgan failure, have occurred with fingolimod in the postmarketing setting. Include disseminated herpetic infections in the differential diagnosis of patients who are receiving TASCENSO ODT and present with an atypical MS relapse or multiorgan failure.
Cases of Kaposi's sarcoma have been reported in patients treated with fingolimod in the postmarketing setting. Kaposi's sarcoma is an angioproliferative disorder that is associated with infection with human herpes virus 8 (HHV-8). Patients with symptoms or signs consistent with Kaposi's sarcoma should be referred for prompt diagnostic evaluation and management.
Cryptococcal Infections
Cryptococcal infections, including cases of fatal cryptococcal meningitis and disseminated cryptococcal infections, have been reported with fingolimod in the postmarketing setting. Cryptococcal infections have generally occurred after approximately 2 years of fingolimod treatment but may occur earlier. The relationship between the risk of cryptococcal infection and the duration of treatment is unknown. Patients with symptoms and signs consistent with a cryptococcal infection should undergo prompt diagnostic evaluation and treatment.
Prior and Concomitant Treatment with Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies
In clinical studies, patients who received fingolimod capsules did not receive concomitant treatment with antineoplastic, non- corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of TASCENSO ODT with any of these therapies, and also with corticosteroids, would be expected to increase the risk of immunosuppression [see Drug Interactions (7.4)].
When switching to fingolimod from immune-modulating or immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects.
Varicella Zoster Virus Antibody Testing/Vaccination
Patients without a healthcare professional confirmed history of chickenpox or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating TASCENSO ODT. VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with TASCENSO ODT, following which initiation of treatment with TASCENSO ODT should be postponed for 1 month to allow the full effect of vaccination to occur [see Drug Interactions (7.3), Use in Specific Populations (8.4)].
Human Papilloma Virus Infection
Human papilloma virus (HPV) infections, including papilloma, dysplasia, warts, and HPV-related cancer, have been reported in patients treated with fingolimod in the postmarketing setting. Vaccination against HPV should be considered prior to treatment initiation with TASCENSO ODT, taking into account vaccination recommendations. Cancer screening, including Papanicolaou (Pap) test, is recommended as per standard of care for patients using an immunosuppressive therapy.
5.3 Progressive Multifocal Leukoencephalopathy
Cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients with MS who received fingolimod, the active moiety in TASCENSO ODT, in the postmarketing setting. PML is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. PML has occurred in patients who had not been treated previously with natalizumab, which has a known association with PML, were not taking any other immunosuppressive or immunomodulatory medications concomitantly and did not have any ongoing systemic medical conditions resulting in compromised immune system function. Longer treatment duration increases the risk of PML in fingolimod-treated patients; the majority of cases have occurred in patients treated with fingolimod for at least 18 months.
At the first sign or symptom suggestive of PML, withhold TASCENSO ODT and perform an appropriate diagnostic evaluation. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
Magnetic resonance imaging (MRI) findings may be apparent before clinical signs or symptoms. Cases of PML, diagnosed based on MRI findings and the detection of JCV DNA in the cerebrospinal fluid in the absence of clinical signs or symptoms specific to PML, have been reported in patients treated with MS medications associated with PML, including fingolimod. Many of these patients subsequently became symptomatic with PML. Therefore, monitoring with MRI for signs that may be consistent with PML may be useful, and any suspicious findings should lead to further investigation to allow for an early diagnosis of PML, if present. Lower PML-related mortality and morbidity have been reported following discontinuation of another MS medication associated with PML in patients with PML who were initially asymptomatic compared to patients with PML who had characteristic clinical signs and symptoms at diagnosis. It is not known whether these differences are due to early detection and discontinuation of MS treatment or due to differences in disease in these patients.
If PML is confirmed, treatment with TASCENSO ODT should be discontinued.
Immune reconstitution inflammatory syndrome (IRIS) has been reported in patients treated with S1P receptor modulators, including fingolimod, who developed PML and subsequently discontinued treatment. IRIS presents as a clinical decline in the patient’s condition that may be rapid, can lead to serious neurological complications or death, and is often associated with characteristic changes on MRI. The time to onset of IRIS in patients with PML was generally within a few months after S1P receptor modulator discontinuation. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken.
5.4 Macular Edema
S1P receptor modulators, including TASCENSO ODT, have been associated with an increased risk of macular edema. Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with TASCENSO ODT. Perform an examination of the fundus, including the macula, 3 to 4 months after starting treatment, periodically while on therapy, and any time there is a change in vision.
A dose-dependent increase in the risk of macular edema occurred in the fingolimod capsules clinical development program.
In 2-year double-blind, placebo-controlled studies in adult patients with multiple sclerosis, macular edema with or without visual symptoms occurred in 1.5% of patients (11/799) treated with fingolimod 1.25 mg capsules, 0.5% of patients (4/783) treated with fingolimod 0.5 mg capsules, and 0.4% of patients (3/773) treated with placebo. Macular edema occurred predominantly during the first 3 to 4 months of therapy. These clinical trials excluded patients with diabetes mellitus, a known risk factor for macular edema (see below Macular Edema in Patients with History of Uveitis or Diabetes Mellitus). Symptoms of macular edema included blurred vision and decreased visual acuity. Routine ophthalmological examination detected macular edema in some patients with no visual symptoms. Macular edema generally partially or completely resolved with or without treatment after drug discontinuation. Some patients had residual visual acuity loss even after resolution of macular edema. Macular edema has also been reported in patients taking fingolimod in the postmarketing setting, usually within the first 6 months of treatment.
Continuation of TASCENSO ODT in patients who develop macular edema has not been evaluated. Macular edema over an extended period of time (i.e., 6 months) can lead to permanent visual loss. Consider discontinuing TASCENSO ODT if macular edema develops; this decision should include an assessment of the potential benefits and risks for the individual patient. The risk of recurrence after rechallenge has not been evaluated.
Macular Edema in Patients with History of Uveitis or Diabetes Mellitus
Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during TASCENSO ODT therapy. In the combined clinical trial experience in adult patients with all doses of fingolimod capsules, the rate of macular edema was higher in MS patients with a history of uveitis compared to those without a history of uveitis (approximately 20% versus 0.6%, respectively). Fingolimod, the active moiety in TASCENSO ODT, has not been tested in MS patients with diabetes mellitus.
5.5 Liver Injury
Clinically significant liver injury has occurred in patients treated with fingolimod in the postmarketing setting. Signs of liver injury, including markedly elevated serum hepatic enzymes and elevated total bilirubin, have occurred as early as ten days after the first dose and have also been reported after prolonged use. Cases of acute liver failure requiring liver transplant have been reported.
In 2-year placebo-controlled clinical trials in adult patients, elevation of liver enzymes (ALT, AST and GGT) to 3-fold the upper limit of normal (ULN) or greater occurred in 14% of patients treated with fingolimod 0.5 mg capsules and 3% of patients on placebo. Elevations 5-fold the ULN or greater occurred in 4.5% of patients on fingolimod capsules and 1% of patients on placebo. The majority of elevations occurred within 6 to 9 months. In clinical trials, fingolimod capsules were discontinued if the elevation exceeded 5 times the ULN. Serum transaminase levels returned to normal within approximately 2 months after discontinuation of fingolimod capsules. Recurrence of liver transaminase elevations occurred with rechallenge in some patients.
Prior to starting treatment with TASCENSO ODT (within 6 months), obtain serum transaminases (ALT and AST) and total bilirubin levels. Obtain transaminase levels and total bilirubin levels periodically until two months after TASCENSO ODT discontinuation.
Patients should be monitored for signs and symptoms of any hepatic injury. Measure liver transaminase and bilirubin levels promptly in patients who report symptoms that may indicate liver injury, including new or worsening fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. In this clinical context, if the patient is found to have an alanine aminotransferase (ALT) greater than three times the reference range with serum total bilirubin greater than two times the reference range, treatment with TASCENSO ODT treatment should be interrupted. Treatment should not be resumed if a plausible alternative etiology for the signs and symptoms cannot be established, because these patients are at risk for severe drug-induced liver injury.
Because fingolimod exposure is doubled in patients with severe hepatic impairment, these patients should be closely monitored during treatment with TASCENSO ODT, as the risk of adverse reactions is greater [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.6 Posterior Reversible Encephalopathy Syndrome
There have been rare cases of posterior reversible encephalopathy syndrome (PRES) reported in adult patients receiving fingolimod. Symptoms reported included sudden onset of severe headache, altered mental status, visual disturbances, and seizure. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, TASCENSO ODT should be discontinued.
5.7 Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) and diffusion lung capacity for carbon monoxide (DLCO) were observed in patients treated with fingolimod, the active moiety in TASCENSO ODT, as early as 1 month after treatment initiation. In 2-year placebo-controlled trials in adult patients, the reduction from baseline in the percent of predicted values for FEV1 at the time of last assessment on drug was 2.8% for fingolimod 0.5 mg capsules and 1.0% for placebo. For DLCO, the reduction from baseline in percent of predicted values at the time of last assessment on drug was 3.3% for fingolimod 0.5 mg capsules and 0.5% for placebo. The changes in FEV1 appear to be reversible after treatment discontinuation. There is insufficient information to determine the reversibility of the decrease of DLCO after drug discontinuation. In MS placebo-controlled trials in adult patients, dyspnea was reported in 9% of patients receiving fingolimod 0.5 mg capsules and 7% of patients receiving placebo. Several patients discontinued fingolimod capsules because of unexplained dyspnea during the extension (uncontrolled) studies. Fingolimod, the active moiety in TASCENSO ODT, has not been tested in MS patients with compromised respiratory function.
Spirometric evaluation of respiratory function and evaluation of DLCO should be performed during therapy with TASCENSO ODT if clinically indicated.
5.8 Fetal Risk
Based on findings from animal studies, TASCENSO ODT may cause fetal harm when administered to a pregnant woman. In animal reproduction studies conducted in rats and rabbits, developmental toxicity was observed with administration of fingolimod at doses less than the recommended human dose. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Because it takes approximately 2 months to eliminate fingolimod from the body, advise females of reproductive potential to use effective contraception to avoid pregnancy during and for 2 months after stopping TASCENSO ODT treatment [see Use in Specific Populations (8.1, 8.3)].
5.9 Severe Increase in Disability After Stopping TASCENSO ODT
Severe increase in disability accompanied by multiple new lesions on MRI has been reported after discontinuation of fingolimod in the postmarketing setting. Patients in most of these reported cases did not return to the functional status they had before stopping fingolimod. The increase in disability generally occurred within 12 weeks after stopping fingolimod but was reported up to 24 weeks after fingolimod discontinuation.
Monitor patients for development of severe increase in disability following discontinuation of TASCENSO ODT and begin appropriate treatment as needed.
After stopping TASCENSO ODT in the setting of PML, monitor for development of immune reconstitution inflammatory syndrome (PML-IRIS) [see Warnings and Precautions (5.3)].
5.10 Tumefactive Multiple Sclerosis
MS relapses with tumefactive demyelinating lesions on imaging have been observed during fingolimod therapy and after fingolimod discontinuation in the postmarketing setting. Most reported cases of tumefactive MS in patients receiving fingolimod have occurred within the first 9 months after fingolimod initiation, but tumefactive MS may occur at any point during treatment. Cases of tumefactive MS have also been reported within the first 4 months after fingolimod discontinuation.
Tumefactive MS should be considered when a severe MS relapse occurs during TASCENSO ODT treatment, especially during initiation, or after discontinuation of TASCENSO ODT, prompting imaging evaluation and initiation of appropriate treatment.
5.11 Increased Blood Pressure
In adult MS controlled clinical trials, patients treated with fingolimod 0.5 mg capsules had an average increase over placebo of approximately 3 mmHg in systolic pressure, and approximately 2 mmHg in diastolic pressure, first detected after approximately 1 month of fingolimod treatment initiation and persisting with continued treatment. Hypertension was reported as an adverse reaction in 8% of patients on fingolimod 0.5 mg capsules and in 4% of patients on placebo. Blood pressure should be monitored during treatment with TASCENSO ODT.
5.12 Malignancies
Cutaneous Malignancies
The risk of cutaneous malignancies (including basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma) is increased in patients treated with S1P receptor modulators. Use of fingolimod, the active moiety in TASCENSO ODT, has been associated with an increased risk of BCC and melanoma.
In two-year placebo-controlled trials in adult patients, the incidence of BCC was 2% in patients on fingolimod 0.5 mg capsules and 1% in patients on placebo [see Adverse Reactions (6.1)]. Melanoma, basal cell carcinoma, squamous cell carcinoma, Kaposi’s sarcoma [see Warnings and Precautions (5.2)], and Merkel cell carcinoma have been reported with fingolimod in the postmarketing setting.
Skin examinations are recommended prior to or shortly after the start of treatment and periodically thereafter for all patients, particularly those with risk factors for skin cancer. Providers and patients are advised to monitor for suspicious skin lesions. If a suspicious skin lesion is observed, it should be promptly evaluated. As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor. Concomitant phototherapy with UV-B radiation or PUVA photochemotherapy is not recommended in patients taking TASCENSO ODT.
Lymphoma
Cases of lymphoma, including both T-cell and B-cell types and CNS lymphoma, have occurred in patients receiving fingolimod, the active moiety in TASCENSO ODT. The reporting rate of non-Hodgkin lymphoma with fingolimod is greater than that expected in the general population adjusted by age, gender, and region. Cutaneous T-cell lymphoma (including mycosis fungoides) has also been reported with fingolimod in the postmarketing setting.
5.13 Immune System Effects Following TASCENSO ODT Discontinuation
Fingolimod remains in the blood and has pharmacodynamic effects, including decreased lymphocyte counts, for up to 2 months following the last dose of TASCENSO ODT. Lymphocyte counts generally return to the normal range within 1 to 2 months of stopping therapy [see Clinical Pharmacology (12.2)]. Because of the continuing pharmacodynamic effects of fingolimod, initiating other drugs during this period warrants the same considerations needed for concomitant administration (e.g., risk of additive immunosuppressant effects) [see Drug Interactions (7.4)].
5.14 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, urticaria, and angioedema have been reported with fingolimod in the postmarketing setting. TASCENSO ODT is contraindicated in patients with history of hypersensitivity to fingolimod or any of its excipients [see Contraindications (4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Bradyarrhythmia and Atrioventricular Blocks [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
- Liver Injury [see Warnings and Precautions (5.5)]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.6)]
- Respiratory Effects [see Warnings and Precautions (5.7)]
- Fetal Risk [see Warnings and Precautions (5.8)]
- Severe Increase in Disability After Stopping TASCENSO ODT [see Warnings and Precautions (5.9)]
- Tumefactive Multiple Sclerosis [see Warnings and Precautions (5.10)]
- Increased Blood Pressure [see Warnings and Precautions (5.11)]
- Malignancies [see Warnings and Precautions (5.12)]
- Immune System Effects Following TASCENSO ODT Discontinuation [see Warnings and Precautions (5.13)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
In clinical trials (Studies 1, 2, and 3), a total of 1212 patients with relapsing forms of multiple sclerosis received fingolimod 0.5 mg capsules. This included 783 patients who received fingolimod 0.5 mg in the 2-year placebo-controlled trials (Studies 1 and 3) and 429 patients who received fingolimod 0.5 mg in the 1-year active-controlled trial (Study 2). The overall exposure in the controlled trials was equivalent to 1716 person-years. Approximately 1000 patients received at least 2 years of treatment with fingolimod 0.5 mg capsules. In all clinical studies, including uncontrolled extension studies, the exposure to fingolimod 0.5 mg capsules was approximately 4119 person-years.
In placebo-controlled trials, the most frequent adverse reactions (incidence ≥ 10% and greater than placebo) for fingolimod 0.5 mg capsules were headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity. Adverse events that led to treatment discontinuation and occurred in more than 1% of patients taking fingolimod 0.5 mg capsules, were serum transaminase elevations (4.7% compared to 1% on placebo) and basal cell carcinoma (1% compared to 0.5% on placebo).
Table 1 lists adverse reactions in clinical studies in adults that occurred in ≥ 1% of fingolimod-treated patients and ≥ 1% higher rate than for placebo.
Table 1: Adverse Reactions Reported in Adult Studies 1 and 3 (Occurring in ≥ 1% of Patients and Reported for Fingolimod 0.5 mg Capsules at ≥ 1% Higher Rate than for Placebo) Fingolimod 0.5 mg Capsules
N = 783Placebo
N = 773Adverse Drug Reactions % % Infections Influenza 11 8 Sinusitis 11 8 Bronchitis 8 5 Herpes zoster 2 1 Tinea versicolor 2 < 1 Cardiac disorders Bradycardia 3 1 Nervous system disorders Headache 25 24 Migraine 6 4 Gastrointestinal disorders Nausea 13 12 Diarrhea 13 10 Abdominal pain 11 10 General disorders and administration-site conditions Asthenia 2 1 Musculoskeletal and connective tissue disorders Back pain 10 9 Pain in extremity 10 7 Skin and subcutaneous tissue disorders Alopecia 3 2 Actinic keratosis 2 1 Investigations Liver transaminase elevations
(ALT/GGT/AST)15 4 Blood triglycerides increased 3 1 Respiratory, thoracic, and mediastinal disorders Cough 12 11 Dyspnea 9 7 Eye disorders Vision blurred 4 2 Vascular disorders Hypertension 8 4 Blood and lymphatic system disorders Lymphopenia 7 < 1 Leukopenia 2 < 1 Neoplasms benign, malignant, and unspecified (including cysts and polyps) Skin papilloma 3 2 Basal cell carcinoma 2 1 Abbreviations: ALT, alanine transaminase; AST, aspartate transferase; GGT, gamma-glutamyl transferase.
Adverse reactions of seizure, dizziness, pneumonia, eczema, and pruritus were also reported in Studies 1 and 3 but did not meet the reporting rate criteria for inclusion in Table 1 (difference was less than 1%).
Adverse reactions with fingolimod 0.5 mg in Study 2, the 1-year active-controlled (versus interferon beta-1a) study were generally similar to those in Studies 1 and 3.
Vascular Events
Vascular events, including ischemic and hemorrhagic strokes, and peripheral arterial occlusive disease were reported in premarketing clinical trials in patients who received fingolimod doses (1.25 mg to 5 mg) higher than recommended for use in MS. Similar events have been reported with fingolimod treatment in the postmarketing setting although a causal relationship has not been established.
Seizure
Cases of seizures, including status epilepticus, have been reported with the use of fingolimod capsules in clinical trials and in the postmarketing setting in adults [see Adverse Reactions (6.2)]. In adult clinical trials, the rate of seizures was 0.9% in fingolimod - treated patients and 0.3% in placebo-treated patients. It is unknown whether these events were related to the effects of multiple sclerosis alone, to fingolimod, or to a combination of both.
Pediatric Patients 10 Years of Age and Older
In the controlled pediatric trial (Study 4), the safety profile in pediatric patients receiving fingolimod 0.25 mg or 0.5 mg capsules daily was similar to that seen in adult patients.
In the pediatric study, cases of seizures were reported in 5.6% of fingolimod-treated patients and 0.9% of interferon beta-1a-treated patients [see Use in Specific Populations (8.4)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fingolimod. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Hemolytic anemia and thrombocytopenia
Hepatobiliary Disorders: Liver injury [see Warnings and Precautions (5.5)]
Infections: Infections including cryptococcal infections [see Warnings and Precautions (5.2)], human papilloma virus (HPV) infection, including papilloma, dysplasia, warts and HPV-related cancer [see Warnings and Precautions (5.2)], progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.3)]
Musculoskeletal and connective tissue disorders: Arthralgia, myalgia
Nervous System Disorders: Posterior reversible encephalopathy syndrome [see Warnings and Precautions (5.6)], seizures, including status epilepticus [see Adverse Reactions (6.1)]
Neoplasms, Benign, Malignant, and Unspecified (including cysts and polyps): melanoma, Merkel cell carcinoma, cutaneous T- cell lymphoma (including mycosis fungoides), Kaposi’s sarcoma and, squamous cell carcinoma [see Warnings and Precautions (5.12)]
Skin and Subcutaneous Tissue Disorders: Hypersensitivity [see Warnings and Precautions (5.14)]
-
7 DRUG INTERACTIONS
7.1 QT Prolonging Drugs
TASCENSO ODT has not been studied in patients treated with drugs that prolong the QT interval. Drugs that prolong the QT interval have been associated with cases of torsades de pointes in patients with bradycardia. Since initiation of TASCENSO ODT treatment results in decreased heart rate and may prolong the QT interval, patients on QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
7.2 Ketoconazole
The blood levels of fingolimod and fingolimod-phosphate are increased by 1.7-fold when used concomitantly with ketoconazole. Patients who use TASCENSO ODT and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater.
7.3 Vaccines
TASCENSO ODT reduces the immune response to vaccination. Vaccination may be less effective during and for up to 2 months after discontinuation of treatment with TASCENSO ODT [see Clinical Pharmacology (12.2)]. Avoid the use of live attenuated vaccines during and for 2 months after treatment with TASCENSO ODT because of the risk of infection.
It is recommended that pediatric patients, if possible, be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating TASCENSO ODT therapy.
7.4 Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies
Antineoplastic, immune-modulating, or immunosuppressive therapies, (including corticosteroids) are expected to increase the risk of immunosuppression, and the risk of additive immune system effects must be considered if these therapies are coadministered with TASCENSO ODT. When switching from drugs with prolonged immune effects, such as natalizumab, teriflunomide or mitoxantrone, the duration and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects when initiating TASCENSO ODT [see Warnings and Precautions (5.2)].
7.5 Drugs That Slow Heart Rate or Atrioventricular Conduction (e.g., beta blockers or diltiazem)
Experience with fingolimod in patients receiving concurrent therapy with drugs that slow the heart rate or AV conduction (e.g., beta blockers, digoxin, or heart rate-slowing calcium channel blockers such as diltiazem or verapamil) is limited. Because initiation of TASCENSO ODT treatment may result in an additional decrease in heart rate, concomitant use of these drugs during TASCENSO ODT initiation may be associated with severe bradycardia or heart block. Seek advice from the physician prescribing these drugs regarding the possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction before initiating TASCENSO ODT. Patients who cannot switch should have overnight continuous ECG monitoring after the first dose [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
7.6 Laboratory Test Interaction
Because fingolimod reduces blood lymphocyte counts via redistribution in secondary lymphoid organs, peripheral blood lymphocyte counts cannot be utilized to evaluate the lymphocyte subset status of a patient treated with fingolimod. A recent CBC should be available before initiating treatment with TASCENSO ODT.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, TASCENSO ODT may cause fetal harm when administered to a pregnant woman. Data from prospective reports to the fingolimod pregnancy registry are currently not sufficient to allow for an adequate assessment of the drug-associated risk for birth defects and miscarriage in humans.
In oral studies conducted in rats and rabbits, fingolimod demonstrated developmental toxicity, including an increase in malformations (rats) and embryolethality, when given to pregnant animals. In rats, the highest no-effect dose was less than the recommended human dose of 0.5 mg/day on a body surface area (mg/m2) basis. The most common fetal visceral malformations in rats were persistent truncus arteriosus and ventricular septal defect. The receptor affected by fingolimod (sphingosine 1-phosphate receptor) is known to be involved in vascular formation during embryogenesis (see Data). Advise pregnant women of the potential risk to a fetus.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
In females planning to become pregnant, TASCENSO ODT should be stopped 2 months before planned conception.
The possibility of severe increase in disability should be considered in women who discontinue or are considering discontinuation of TASCENSO ODT because of pregnancy or planned pregnancy. In many of the cases in which increase in disability was reported after stopping fingolimod, the active moiety in TASCENSO ODT, patients had stopped fingolimod because of pregnancy or planned pregnancy [see Warnings and Precautions (5.9].
Data
Animal Data
When fingolimod was orally administered to pregnant rats during the period of organogenesis (0, 0.03, 0.1, and 0.3 mg/kg/day or 0, 1, 3, and 10 mg/kg/day), increased incidences of fetal malformations and embryofetal deaths were observed at all but the lowest dose tested (0.03 mg/kg/day), which is less than the recommended human dose (RHD) on a mg/m2 basis. Oral administration to pregnant rabbits during organogenesis (0, 0.5, 1.5, and 5 mg/kg/day) resulted in increased incidences of embryofetal mortality and fetal growth retardation at the mid and high doses. The no-effect dose for these effects in rabbits (0.5 mg/kg/day) is approximately 20 times the RHD on a mg/m2 basis.
When fingolimod was orally administered to female rats during pregnancy and lactation (0, 0.05, 0.15, and 0.5 mg/kg/day), pup survival was decreased at all doses and a neurobehavioral (learning) deficit was seen in offspring at the high dose. The low-effect dose of 0.05 mg/kg/day is similar to the RHD on a mg/m2 basis.8.2 Lactation
Risk Summary
There are no data on the presence of fingolimod in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Fingolimod is excreted in the milk of treated rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TASCENSO ODT and any potential adverse effects on the breastfed infant from TASCENSO ODT or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
The pregnancy status of females of reproductive potential should be verified prior to starting treatment with TASCENSO ODT [see Use in Specific Populations (8.1)].
Contraception
Before initiation of TASCENSO ODT, females of reproductive potential should be counseled on the potential for a serious risk to the fetus and the need for effective contraception during treatment with TASCENSO ODT [see Warnings and Precautions (5.9) and Use in Specific Populations (8.1)]. Since it takes approximately 2 months to eliminate the compound from the body after stopping treatment, the potential risk to the fetus may persist and women should use effective contraception during this period [see Warnings and Precautions (5.8, 5.13)].
8.4 Pediatric Use
Safety and effectiveness of fingolimod for the treatment of relapsing forms of multiple sclerosis in pediatric patients 10 to less than 18 years of age were established in one randomized, double-blind clinical study in 215 patients (fingolimod n = 107; intramuscular interferon (IFN) beta-1a n = 108) [see Clinical Studies (14.2)].
In the controlled pediatric study, the safety profile in pediatric patients (10 to less than 18 years of age) receiving fingolimod 0.25 mg or 0.5 mg capsules daily was similar to that seen in adult patients. In the pediatric study, cases of seizures were reported in 5.6% of fingolimod-treated patients and 0.9% of interferon beta-1a-treated patients.
It is recommended that pediatric patients, if possible, complete all immunizations in accordance with current immunization guidelines prior to initiating TASCENSO ODT therapy.
Safety and effectiveness of TASCENSO ODT in pediatric patients below the age of 10 years have not been established.
Juvenile Animal Toxicity Data
In a study in which fingolimod (0.3, 1.5, or 7.5 mg/kg/day) was orally administered to young rats from weaning through sexual maturity, changes in bone mineral density and persistent neurobehavioral impairment (altered auditory startle) were observed at all doses. Delayed sexual maturation was noted in females at the highest dose tested and in males at all doses. The bone changes observed in fingolimod-treated juvenile rats are consistent with a reported role of S1P in the regulation of bone mineral homeostasis.
When fingolimod (0.5 or 5 mg/kg/day) was orally administered to rats from the neonatal period through sexual maturity, a marked decrease in T-cell dependent antibody response was observed at both doses. This effect had not fully recovered by 6 to 8 weeks after the end of treatment.
Overall, a no-effect dose for adverse developmental effects in juvenile animals was not identified.
8.5 Geriatric Use
Clinical MS studies of fingolimod capsules did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. TASCENSO ODT should be used with caution in patients aged 65 years and over, reflecting the greater frequency of decreased hepatic, or renal, function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Because fingolimod, but not fingolimod-phosphate, exposure is doubled in patients with severe hepatic impairment, patients with severe hepatic impairment should be closely monitored, as the risk of adverse reactions may be greater [see Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
No dose adjustment is needed in patients with mild or moderate hepatic impairment.
8.7 Renal Impairment
The blood level of some fingolimod metabolites is increased (up to 13-fold) in patients with severe renal impairment [see Clinical Pharmacology (12.3)]. The toxicity of these metabolites has not been fully explored. The blood level of these metabolites has not been assessed in patients with mild or moderate renal impairment.
-
10 OVERDOSAGE
Fingolimod, the active moiety in TASCENSO ODT, can induce bradycardia as well as AV conduction blocks (including complete AV block). The decline in heart rate usually starts within 1 hour of the first dose and is maximal within 6 hours in most patients [see Warnings and Precautions (5.1)]. In case of TASCENSO ODT overdosage, observe patients overnight with continuous ECG monitoring in a medical facility, and obtain regular measurements of blood pressure. [see Dosage and Administration (2.4)].
Neither dialysis nor plasma exchange results in removal of fingolimod from the body.
-
11 DESCRIPTION
Fingolimod is a sphingosine 1-phosphate receptor modulator.
Chemically, fingolimod lauryl sulfate is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol lauryl sulfate. Its structure is shown below:
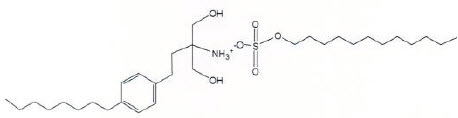
Fingolimod lauryl sulfate is a white to practically white powder that is practically insoluble in water. It has a molecular weight of 573.87 g/mol.
TASCENSO ODT is provided as 0.25 mg and 0.5 mg orally disintegrating tablets for oral use.
Each orally disintegrating tablet contains 0.25 mg or 0.5 mg of fingolimod (equivalent to 0.47 mg or 0.93 mg of fingolimod lauryl sulfate) and the following inactive ingredients: gelatin, mannitol, medium-chain triglycerides, sodium chloride, and sodium lauryl sulfate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fingolimod is metabolized by sphingosine kinase to the active metabolite, fingolimod-phosphate. Fingolimod phosphate is a sphingosine 1-phosphate receptor modulator and binds with high affinity to sphingosine 1-phosphate receptors 1, 3, 4, and 5. Fingolimod-phosphate blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which fingolimod exerts therapeutic effects in multiple sclerosis is unknown but may involve reduction of lymphocyte migration into the central nervous system.
12.2 Pharmacodynamics
Heart Rate and Rhythm
Fingolimod causes a transient reduction in heart rate and AV conduction at treatment initiation [see Warnings and Precautions (5.1)].
Heart rate progressively increases after the first day, returning to baseline values within 1 month of the start of chronic treatment.
Autonomic responses of the heart, including diurnal variation of heart rate and response to exercise, are not affected by fingolimod treatment.
Fingolimod treatment is not associated with a decrease in cardiac output.
Potential to Prolong the QT Interval
In a thorough QT interval study of doses of 1.25 or 2.5 mg fingolimod capsules at steady-state, when a negative chronotropic effect of fingolimod was still present, fingolimod treatment resulted in a prolongation of QTc, with the upper boundary of the 90% confidence interval (CI) of 14.0 msec. There is no consistent signal of increased incidence of QTc outliers, either absolute or change from baseline, associated with fingolimod treatment. In MS studies, there was no clinically relevant prolongation of the QT interval, but patients at risk for QT prolongation were not included in clinical studies.
Immune System
Effects on Immune Cell Numbers in the Blood
In a study in which 12 adult subjects received fingolimod 0.5 mg capsules daily, the lymphocyte count decreased to approximately 60% of baseline within 4 to 6 hours after the first dose. With continued daily dosing, the lymphocyte count continued to decrease over a 2-week period, reaching a nadir count of approximately 500 cells/mcL or approximately 30% of baseline. In a placebo-controlled study in 1272 MS patients (of whom 425 received fingolimod 0.5 mg capsules daily and 418 received placebo), 18% (N = 78) of patients on fingolimod 0.5 mg reached a nadir of < 200 cells/mcL on at least 1 occasion. No patient on placebo reached a nadir of < 200 cells/mcL. Low lymphocyte counts are maintained with chronic daily dosing of fingolimod 0.5 mg daily.
Chronic fingolimod dosing leads to a mild decrease in the neutrophil count to approximately 80% of baseline. Monocytes are unaffected by fingolimod.
Peripheral lymphocyte count increases are evident within days of stopping fingolimod treatment and typically normal counts are reached within 1 to 2 months.
Effect on Antibody Response
Fingolimod reduces the immune response to vaccination, as evaluated in 2 studies.
In the first study, the immunogenicity of keyhole limpet hemocyanin (KLH) and pneumococcal polysaccharide vaccine (PPV-23) immunization were assessed by IgM and IgG titers in a steady-state, randomized, placebo-controlled study in healthy adult volunteers. Compared to placebo, antigen-specific IgM titers were decreased by 91% and 25% in response to KLH and PPV-23, respectively, in subjects on fingolimod 0.5 mg capsules. Similarly, IgG titers were decreased by 45% and 50%, in response to KLH and PPV-23, respectively, in subjects on fingolimod 0.5 mg daily compared to placebo. The responder rate for fingolimod 0.5 mg as measured by the number of subjects with a > 4-fold increase in KLH IgG was comparable to placebo and 25% lower for PPV-23 IgG, while the number of subjects with a > 4-fold increase in KLH and PPV-23 IgM was 75% and 40% lower, respectively, compared to placebo. The capacity to mount a skin delayed-type hypersensitivity reaction to Candida and tetanus toxoid was decreased by approximately 30% in subjects on fingolimod 0.5 mg daily, compared to placebo. Immunologic responses were further decreased with fingolimod 1.25 mg (a dose higher than recommended in MS) [see Warnings and Precautions (5.2)].
In the second study, the immunogenicity of Northern hemisphere seasonal influenza and tetanus toxoid vaccination was assessed in a 12-week steady-state, randomized, placebo-controlled study of fingolimod 0.5 mg capsules in adult multiple sclerosis patients (n = 136). The responder rate 3 weeks after vaccination, defined as seroconversion or a ≥ 4-fold increase in antibody directed against at least 1 of the 3 influenza strains, was 54% for fingolimod 0.5 mg and 85% in the placebo group. The responder rate 3 weeks after vaccination, defined as seroconversion or a ≥ 4-fold increase in antibody directed against tetanus toxoid was 40% for fingolimod 0.5 mg and 61% in the placebo group.
Pulmonary Function
Single fingolimod doses ≥ 5 mg (10-fold the recommended dose) are associated with a dose-dependent increase in airway resistance. In a 14-day study of 0.5, 1.25, or 5 mg/day, fingolimod was not associated with impaired oxygenation or oxygen desaturation with exercise or an increase in airway responsiveness to methacholine. Subjects on fingolimod treatment had a normal bronchodilator response to inhaled beta-agonists.
In a 14-day placebo-controlled study of adult patients with moderate asthma, no effect was seen for fingolimod 0.5 mg capsules (recommended dose in MS). A 10% reduction in mean FEV1 at 6 hours after dosing was observed in adult patients receiving fingolimod 1.25 mg (a dose higher than recommended for use in MS) on Day 10 of treatment. Fingolimod 1.25 mg was associated with a 5-fold increase in the use of rescue short-acting beta-agonists.
12.3 Pharmacokinetics
Absorption
The Tmax of fingolimod is 12 to 16 hours. The apparent absolute oral bioavailability is 93%.
Food intake does not alter Cmax or (AUC) of fingolimod or fingolimod-phosphate. Therefore, TASCENSO ODT may be taken without regard to meals.
Steady-state blood concentrations of fingolimod are reached within 1 to 2 months following once-daily administration and steady-state levels are approximately 10-fold greater than with the initial dose.
Distribution
Fingolimod highly (86%) distributes in red blood cells. Fingolimod-phosphate has a smaller uptake in blood cells of < 17%. Fingolimod and fingolimod-phosphate are > 99.7% protein bound. Fingolimod and fingolimod-phosphate protein binding is not altered by renal or hepatic impairment.
Fingolimod is extensively distributed to body tissues with a volume of distribution of about 1200 ± 260 L.
Metabolism
The biotransformation of fingolimod in humans occurs by 3 main pathways: by reversible stereoselective phosphorylation to the pharmacologically active (S)-enantiomer of fingolimod-phosphate, by oxidative biotransformation catalyzed mainly by the cytochrome P450 4F2 (CYP4F2) and possibly other CYP4F isoenzymes with subsequent fatty acid-like degradation to inactive metabolites, and by formation of pharmacologically inactive non-polar ceramide analogs of fingolimod.
Inhibitors or inducers of CYP4F2 and possibly other CYP4F isozymes might alter the exposure of fingolimod or fingolimod-phosphate. In vitro studies in hepatocytes indicated that CYP3A4 may contribute to fingolimod metabolism in the case of strong induction of CYP3A4.
Following single oral administration of [14C] fingolimod, the major fingolimod-related components in blood, as judged from their contribution to the AUC up to 816 hours post-dose of total radiolabeled components, are fingolimod itself (23.3%), fingolimod-phosphate (10.3%), and inactive metabolites [M3 carboxylic acid metabolite (8.3%), M29 ceramide metabolite (8.9%), and M30 ceramide metabolite (7.3%)].
Elimination
Fingolimod blood clearance is 6.3 ± 2.3 L/h, and the average apparent terminal half-life (t1/2) is 6 to 9 days. Blood levels of fingolimod-phosphate decline in parallel with those of fingolimod in the terminal phase, yielding similar half-lives for both.
After oral administration, about 81% of the dose is slowly excreted in the urine as inactive metabolites. Fingolimod and fingolimod-phosphate are not excreted intact in urine but are the major components in the feces with amounts of each representing less than 2.5% of the dose.
Specific Populations
Pediatric Patients
The median fingolimod-phosphate (fingolimod-P) concentration in pediatric MS patients aged 10 to less than 18 years was 1.10 ng/mL, as compared to 1.35 ng/mL in adult MS patients.
Geriatric Patients
The mechanism for elimination and results from population pharmacokinetics suggest that dose adjustment would not be necessary in elderly patients. However, clinical experience in patients aged above 65 years is limited.Gender
Gender has no clinically significant influence on fingolimod and fingolimod-phosphate pharmacokinetics.
Race
The effects of race on fingolimod and fingolimod-phosphate pharmacokinetics cannot be adequately assessed due to a low number of patients who self-identified as Black or African American, Asian, or other races in the clinical program.
Renal Impairment
In adult patients with severe renal impairment, fingolimod Cmax and AUC are increased by 32% and 43%, respectively, and fingolimod-phosphate Cmax and AUC are increased by 25% and 14%, respectively, with no change in apparent elimination half-life. Based on these findings, the TASCENSO ODT 0.5 mg dose is appropriate for use in adult patients with renal impairment. TASCENSO ODT 0.25 mg and 0.5 mg are appropriate for use in pediatric patients with renal impairment. The systemic exposure of 2 metabolites (M2 and M3) is increased by 3-and 13-fold, respectively. The toxicity of these metabolites has not been fully characterized.
A study in patients with mild or moderate renal impairment has not been conducted.
Hepatic Impairment
In subjects with mild, moderate, or severe hepatic impairment (Child-Pugh class A, B, and C), no change in fingolimod Cmax was observed, but fingolimod AUC0-∞ was increased respectively by 12%, 44%, and 103%. In patients with severe hepatic impairment (Child-Pugh class C), fingolimod-phosphate Cmax was decreased by 22% and AUC0-96 hours was decreased by 29%. The pharmacokinetics of fingolimod-phosphate was not evaluated in patients with mild or moderate hepatic impairment. The apparent elimination half-life of fingolimod is unchanged in subjects with mild hepatic impairment but is prolonged by about 50% in patients with moderate or severe hepatic impairment.
Patients with severe hepatic impairment (Child-Pugh class C) should be closely monitored, as the risk of adverse reactions is greater [see Warnings and Precautions (5.6)].
No dose adjustment is needed in patients with mild or moderate hepatic impairment (Child-Pugh class A and B).
Drug Interactions
Ketoconazole
The coadministration of ketoconazole (a potent inhibitor of CYP3A and CYP4F) 200 mg twice-daily at steady-state and a single dose of fingolimod 5 mg led to a 70% increase in AUC of fingolimod and fingolimod-phosphate. Patients who use TASCENSO ODT and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater [see Drug Interactions (7.2)].
Carbamazepine
The coadministration of carbamazepine (a potent CYP450 enzyme inducer) 600 mg twice-daily at steady-state and a single dose of fingolimod 2 mg decreased blood concentrations (AUC) of fingolimod and fingolimod-phosphate by approximately 40%. The clinical impact of this decrease is unknown.
Other strong CYP450 enzyme inducers, e.g., rifampicin, phenytoin, phenobarbital, and St. John's wort, may also reduce AUC of fingolimod and fingolimod-phosphate. The clinical impact of this potential decrease is unknown.
Potential of Fingolimod and Fingolimod-phosphate to Inhibit the Metabolism of Comedications
In vitro inhibition studies using pooled human liver microsomes and specific metabolic probe substrates demonstrate that fingolimod has little or no capacity to inhibit the activity of the following CYP enzymes: CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or CYP4A9/11 (fingolimod only), and similarly fingolimod-phosphate has little or no capacity to inhibit the activity of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 at concentrations up to 3 orders of magnitude of therapeutic concentrations. Therefore, fingolimod and fingolimod-phosphate are unlikely to reduce the clearance of drugs that are mainly cleared through metabolism by the major CYP isoenzymes described above.
Potential of Fingolimod and Fingolimod-phosphate to Induce its Own and/or the Metabolism of Comedications
Fingolimod was examined for its potential to induce human CYP3A4, CYP1A2, CYP4F2, and MDR1 (P-glycoprotein) mRNA and CYP3A, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP4F2 activity in primary human hepatocytes. Fingolimod did not induce mRNA or activity of the different CYP enzymes and MDR1 with respect to the vehicle control; therefore, no clinically relevant induction of the tested CYP enzymes or MDR1 by fingolimod are expected at therapeutic concentrations. Fingolimod-phosphate was also examined for its potential to induce mRNA and/or activity of human CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A, CYP4F2, CYP4F3B, and CYP4F12. Fingolimod-phosphate is not expected to have clinically significant induction effects on these enzymes at therapeutic doses of fingolimod. In vitro experiments did not provide an indication of CYP induction by fingolimod-phosphate.
Transporters
Based on in vitro data, fingolimod as well as fingolimod-phosphate are not expected to inhibit the uptake of comedications and/or biologics transported by the organic anion transporting polypeptides OATP1B1, OATP1B3, or the sodium taurocholate co-transporting polypeptide (NTCP). Similarly, they are not expected to inhibit the efflux of comedications and/or biologics transported by the breast cancer resistance protein (BCRP), the bile salt export pump (BSEP), the multidrug resistance-associated protein 2 (MRP2), or P-glycoprotein (P-gp) at therapeutic concentrations.
Oral Contraceptives
The coadministration of fingolimod 0.5 mg capsules daily with oral contraceptives (ethinylestradiol and levonorgestrel) did not elicit any clinically significant change in oral contraceptives exposure. Fingolimod and fingolimod-phosphate exposure were consistent with those from previous studies. No interaction studies have been performed with oral contraceptives containing other progestagens; however, an effect of fingolimod on their exposure is not expected.
Cyclosporine
The pharmacokinetics of single-dose fingolimod was not altered during coadministration with cyclosporine at steady-state, nor was cyclosporine steady-state pharmacokinetics altered by fingolimod. These data indicate that TASCENSO ODT is unlikely to reduce or increase the clearance of drugs cleared mainly by CYP3A4. Potent inhibition of transporters MDR1 (P-gp), MRP2, and OATP-1B1 does not influence fingolimod disposition.
Isoproterenol, Atropine, Atenolol, and Diltiazem
Single-dose fingolimod and fingolimod-phosphate exposure was not altered by coadministered isoproterenol or atropine. Likewise, the single-dose pharmacokinetics of fingolimod and fingolimod-phosphate and the steady-state pharmacokinetics of both atenolol and diltiazem were unchanged during the coadministration of the latter 2 drugs individually with fingolimod.
Population Pharmacokinetics Analysis
A population pharmacokinetics evaluation performed in MS patients did not provide evidence for a significant effect of fluoxetine and paroxetine (strong CYP2D6 inhibitors) on fingolimod or fingolimod-phosphate predose concentrations. In addition, the following commonly coprescribed substances had no clinically relevant effect (< 20%) on fingolimod or fingolimod-phosphate predose concentrations: baclofen, gabapentin, oxybutynin, amantadine, modafinil, amitriptyline, pregabalin, and corticosteroids.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies of fingolimod were conducted in mice and rats. In mice, fingolimod was administered at oral doses of 0, 0.025, 0.25, and 2.5 mg/kg/day for up to 2 years. The incidence of malignant lymphoma was increased in males and females at the mid and high dose. The lowest dose tested (0.025 mg/kg/day) is less than the RHD of 0.5 mg/day on a body surface area (mg/m2) basis. In rats, fingolimod was administered at oral doses of 0, 0.05, 0.15, 0.5, and 2.5 mg/kg/day. No increase in tumors was observed. The highest dose tested (2.5 mg/kg/day) is approximately 50 times the RHD on a mg/m2 basis.
Fingolimod was negative in a battery of in vitro (Ames, mouse lymphoma thymidine kinase, chromosomal aberration in mammalian cells) and in vivo (micronucleus in mouse and rat) assays.When fingolimod was administered orally (0, 1, 3, and 10 mg/kg/day) to male and female rats prior to and during mating, and continuing to Day 7 of gestation in females, no effect on fertility was observed up to the highest dose tested (10 mg/kg), which is approximately 200 times the RHD on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
Lung toxicity was observed in 2 different strains of rats and in dogs and monkeys. The primary findings included increase in lung weight, associated with smooth muscle hypertrophy, hyperdistention of the alveoli, and/or increased collagen. Insufficient or lack of pulmonary collapse at necropsy, generally correlated with microscopic changes, was observed in all species. In rats and monkeys, lung toxicity was observed at all oral doses tested in chronic studies. The lowest doses tested in rats (0.05 mg/kg/day in the 2-year carcinogenicity study) and monkeys (0.5 mg/kg/day in the 39-week toxicity study) are similar to and approximately 20 times the RHD on a mg/m2 basis, respectively.
In the 52-week oral study in monkeys, respiratory distress associated with ketamine administration was observed at doses of 3 and 10 mg/kg/day; the most affected animal became hypoxic and required oxygenation. As ketamine is not generally associated with respiratory depression, this effect was attributed to fingolimod. In a subsequent study in rats, ketamine was shown to potentiate the bronchoconstrictive effects of fingolimod. The relevance of these findings to humans is unknown.
-
14 CLINICAL STUDIES
The efficacy of TASCENSO ODT is based on the relative bioavailability of TASCENSO ODT orally disintegrating tablets compared to fingolimod capsules in healthy adults [see Clinical Pharmacology (12.3)].
The clinical studies described below were conducted using fingolimod capsules.
14.1 Adults
The efficacy of fingolimod was demonstrated in 2 studies that evaluated once-daily doses of fingolimod capsules 0.5 mg and 1.25 mg in patients with relapsing-remitting MS (RRMS). Both studies included patients who had experienced at least 2 clinical relapses during the 2 years prior to randomization or at least 1 clinical relapse during the 1 year prior to randomization and had an Expanded Disability Status Scale (EDSS) score from 0 to 5.5. Study 1 was a 2-year randomized, double-blind, placebo-controlled study in patients with RRMS who had not received any interferon-beta or glatiramer acetate for at least the previous 3 months and had not received any natalizumab for at least the previous 6 months. Neurological evaluations were performed at screening, every 3 months and at time of suspected relapse. MRI evaluations were performed at screening, Month 6, Month 12, and Month 24. The primary endpoint was the annualized relapse rate.
Median age was 37 years, median disease duration was 6.7 years and median EDSS score at baseline was 2.0. Patients were randomized to receive fingolimod 0.5 mg (N = 425), 1.25 mg (N = 429), or placebo (N = 418) for up to 24 months. Median time on study drug was 717 days on 0.5 mg, 715 days on 1.25 mg, and 719 days on placebo.
The annualized relapse rate was significantly lower in patients treated with fingolimod than in patients who received placebo. The secondary endpoint was the time to 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5) sustained for 3 months. Time to onset of 3-month confirmed disability progression was significantly delayed with fingolimod treatment compared to placebo. The 1.25 mg dose resulted in no additional benefit over the fingolimod 0.5 mg dose. The results for this study are shown in Table 2 and Figure 1.
Table 2: Clinical and MRI Results of Study 1 Fingolimod
Capsules 0.5 mgPlacebo p-value N = 425 N = 418 Clinical Endpoints Annualized relapse rate (primary endpoint) 0.18 0.40 < 0.001 Percentage of patients without relapse 70% 46% < 0.001 Hazard ratio§ of disability progression
(95% CI)0.70
(0.52, 0.96)0.02 MRI Endpoint Mean (median) number of new or newly enlarging T2 lesions over 24 months 2.5 (0) 9.8 (5.0) < 0.001 Mean (median) number of T1 Gd-enhancing lesions at Month 24 0.2 (0) 1.1 (0) < 0.001 Abbreviation: CI, confidence interval.
All analyses of clinical endpoints were intent-to-treat. MRI analysis used evaluable dataset.
§Hazard ratio is an estimate of the relative risk of having the event of disability progression on fingolimod as compared to placebo.Figure 1: Time to 3-Month Confirmed Disability Progression - Study 1 (ITT population)
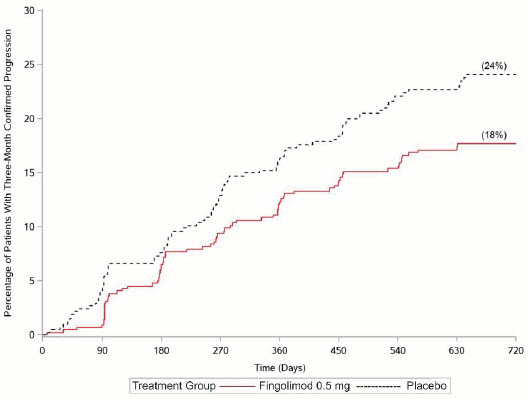
Study 2 was a 1-year randomized, double-blind, double-dummy, active-controlled study in patients with RRMS who had not received any natalizumab in the previous 6 months. Prior therapy with interferon-beta or glatiramer acetate up to the time of randomization was permitted.
Neurological evaluations were performed at screening, every 3 months, and at the time of suspected relapses. MRI evaluations were performed at screening and at Month 12. The primary endpoint was the annualized relapse rate.
Median age was 36 years, median disease duration was 5.9 years, and median EDSS score at baseline was 2.0. Patients were randomized to receive fingolimod capsules 0.5 mg (N = 431), 1.25 mg (N = 426), or interferon beta-1a, 30 mcg via the intramuscular route (IM) once-weekly (N = 435) for up to 12 months. Median time on study drug was 365 days on fingolimod 0.5 mg, 354 days on 1.25 mg, and 361 days on interferon beta-1a IM.
The annualized relapse rate was significantly lower in patients treated with fingolimod 0.5 mg than in patients who received interferon beta-1a IM. The key secondary endpoints were number of new and newly enlarging T2 lesions and time to onset of 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5-point increase for those with baseline EDSS of 5.5) sustained for 3 months. The number of new and newly enlarging T2 lesions was significantly lower in patients treated with fingolimod than in patients who received interferon beta-1a IM. There was no significant difference in the time to 3-month confirmed disability progression between fingolimod and interferon beta-1a-treated patients at 1 year. The 1.25 mg dose resulted in no additional benefit over the fingolimod 0.5 mg dose. The results for this study are shown in Table 3.
Table 3: Clinical and MRI Results of Study 2 Fingolimod
CapsulesInterferon beta-1a p-value 0.5 mg IM 30 mcg N = 429 N = 431 Clinical Endpoints Annualized relapse rate (primary endpoint) 0.16 0.33 < 0.001 Percentage of patients without relapse 83% 70% < 0.001 Hazard ratio§of disability progression
(95% CI)0.71
(0.42, 1.21)0.21 MRI Endpoint Mean (median) number of new or newly enlarging T2 lesions over 12 months 1.6 (0) 2.6 (1.0) 0.002 Mean (median) number of T1 Gd enhancing lesions at Month 12 0.2 (0) 0.5 (0) < 0.001 Abbreviation: CI, confidence interval.
All analyses of clinical endpoints were intent-to-treat. MRI analysis used evaluable dataset.
§Hazard ratio is an estimate of the relative risk of having the event of disability progression on fingolimod as compared to control.
Pooled results of Study 1 and Study 2 showed a consistent and statistically significant reduction of annualized relapse rate compared to comparator in subgroups defined by gender, age, prior MS therapy, and disease activity.
14.2 Pediatric Patients (10 to less than 18 Years of Age)
Study 4 (NCT01892722) evaluated the efficacy of once-daily oral doses of fingolimod capsules 0.25 mg or fingolimod capsules 0.5 mg in pediatric patients 10 to less than 18 years of age with relapsing-remitting multiple sclerosis. Study 4 was a 215 patient, double-blind, randomized, clinical trial that compared fingolimod to intramuscular interferon beta-1a. Prior therapy with interferon-beta, dimethyl fumarate, or glatiramer acetate up to the time of randomization was permitted. The study included patients who had experienced at least 1 clinical relapse during the year prior or 2 relapses during the 2 years prior to screening, or evidence of 1 or more Gd-enhancing lesions on MRI within 6 months prior to randomization and had an EDSS score from 0 to 5.5. Neurological evaluations were scheduled at screening, every 3 months, and at the time of suspected relapses. MRI evaluations were performed at screening and every 6 months throughout the study. The primary endpoint was the annualized relapse rate.
At baseline, the median age was 16 years, median disease duration since first symptom was 1.5 years, and median EDSS score was 1.5. One patient received no study drug and is excluded from the analysis of efficacy. Median duration of exposure to study drug was 634 days in the fingolimod group (n = 107) and 547 days in the interferon beta-1a group (n = 107). In the fingolimod group, 6.5% of patients did not complete the study, compared to 18.5% in the interferon beta-1a group.
The primary endpoint, the annualized relapse rate (ARR), was significantly lower in patients treated with fingolimod (0.122) than in patients who received interferon beta-1a (0.675). Relative reduction in ARR was 81.9%. The annualized rate of the number of new or newly enlarged T2 lesions up to month 24 (key secondary endpoint) was significantly lower in patients treated with fingolimod, as was the number of Gd-enhancing T1 lesions per scan up to month 24.
Table 4 summarizes the results of Study 4.
Table 4: Clinical and MRI Results of Study 4 Fingolimod
0.25 mg or 0.5 mg PO
N = 107Interferon beta-1a
30 mcg IM
N = 107p-value Relative
ReductionClinical endpoints Annualized relapse rate (primary endpoint) 0.122 0.675 < 0.001§ 81.9% Percent of patients remaining relapse-free at 24 months 86.0% 45.8% MRI endpoints Annualized rate of the number of new or newly enlarging T2 lesions 4.393 9.269 < 0.001§ 52.6% Mean number of Gd-enhancing T1 lesions per scan up to Month 24 0.436 0.436 < 0.001§ 66.0% Abbreviations: IM, intramuscular; MRI, magnetic resonance imaging; PO, by mouth.
All analyses of clinical endpoints were on full analysis set. MRI analyses used the evaluable dataset.
§Indicates statistical significance vs. Interferon beta-1a IM at two-sided 0.05 level.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
0.25 mg TASCENSO ODT orally disintegrating tablets are supplied as follows:
White to off-white, round, orally disintegrating tablet debossed with .
.Carton of 30 orally disintegrating tablets containing 3 blister cards of 10 orally disintegrating tablets per blister card
NDC: 70709-062-30
0.5 mg TASCENSO ODT orally disintegrating tablets are supplied as follows:White to off-white, round, orally disintegrating tablet debossed with
 .
.Carton of 30 orally disintegrating tablets containing 3 blister cards of 10 orally disintegrating tablets per blister card
NDC: 70709-065-30
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration
Tell patients not to discontinue TASCENSO ODT without first discussing this with the prescribing healthcare provider. Advise patients to contact their healthcare provider if they accidently take more TASCENSO ODT than prescribed. Advise patients to use dry hands when opening the blister pack. Instruct patients to not push the ODT through the lidding foil, but to peel back the lidding foil and then push the underside [see Dosage and Administration (2.2)].
Cardiac Effects
Advise patients that initiation of TASCENSO ODT treatment results in a transient decrease in heart rate. Inform patients that they will need to be observed in the healthcare professional's office or other facility for at least 6 hours after the first dose, after reinitiation if treatment is interrupted or discontinued for certain periods, and after the dosage is increased [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
Risk of Infections
Inform patients that they may have an increased risk of infections, some of which could be life-threatening, when taking TASCENSO ODT, and that they should contact their healthcare provider if they develop symptoms of infection. Advise patients that the use of some vaccines should be avoided during treatment with TASCENSO ODT and for 2 months after discontinuation. Recommend to patients that they delay treatment with TASCENSO ODT until after VZV vaccination if they have not had chickenpox or a previous VZV vaccination. Inform patients that prior or concomitant use of drugs that suppress the immune system may increase the risk of infection [see Warnings and Precautions (5.2)].
Progressive Multifocal Leukoencephalopathy
Inform patients that cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients who received fingolimod, the active moiety in TASCENSO ODT. Inform the patient that PML is characterized by a progression of deficits and usually leads to death or severe disability over weeks or months. Instruct the patient of the importance of contacting their healthcare provider if they develop any symptoms suggestive of PML. Inform the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes [see Warnings and Precautions (5.3)].
Macular Edema
Advise patients that TASCENSO ODT may cause macular edema, and that they should obtain an eye exam near the start of treatment with TASCENSO ODT, have their eyes monitored periodically by an eye care professional while receiving therapy, and contact their healthcare provider if they experience any changes in their vision while taking TASCENSO ODT. Inform patients with diabetes mellitus or a history of uveitis that their risk of macular edema is increased [see Warnings and Precautions (5.4)].
Hepatic Effects
Inform patients that TASCENSO ODT may cause liver injury. Advise patients that they should contact their healthcare provider if they have any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine [see Warnings and Precautions (5.5)].
Posterior Reversible Encephalopathy Syndrome
Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological sequelae [see Warnings and Precautions (5.6)].
Respiratory Effects
Advise patients that they should contact their healthcare provider if they experience new onset or worsening of dyspnea [see Warnings and Precautions (5.7)].
Fetal Risk
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1, 8.3)].
- Advise female patients of reproductive potential to use effective contraception during treatment with TASCENSO ODT and for two months after the final dose [see Use in Specific Populations (8.3)].
Severe Increase in Disability After Stopping TASCENSO ODT
Inform patients that severe increase in disability has been reported after discontinuation of fingolimod. Advise patients to contact their healthcare provider if they develop worsening symptoms of MS following discontinuation of TASCENSO ODT [see Warnings and Precautions (5.9)].
Malignancies
Advise patients that the risk of basal cell carcinoma and melanoma is increased with use of fingolimod, the active moiety in TASCENSO ODT, and that cases of squamous cell carcinoma, Merkel cell carcinoma, and Kaposi’s sarcoma have been reported. Advise patients that any suspicious skin lesions should be promptly evaluated. Advise patients to limit exposure to sunlight and ultraviolet light by wearing protective clothing and using a sunscreen with a high protection factor. Inform patients that lymphoma has also occurred in patients receiving fingolimod [see Warnings and Precautions (5.12)].
Persistence of TASCENSO ODT Effects After Drug Discontinuation
Advise patients that TASCENSO ODT remains in the blood and continues to have effects, including decreased blood lymphocyte counts, for up to 2 months following the last dose [see Warnings and Precautions (5.13)].
Hypersensitivity Reactions
Advise patients that TASCENSO ODT may cause hypersensitivity reactions including rash, urticaria, and angioedema. Advise patients to contact their healthcare provider if they have any symptoms associated with hypersensitivity [see Warnings and Precautions (5.14)].
Pregnancy
Instruct patients that if they are pregnant or plan to become pregnant while taking TASCENSO ODT they should inform their physician [see Use in Specific Populations (8.1)].
For full prescribing information, please visit www.tascenso.com.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Catalent Pharma Solutions,
Frankland Road, Balgrove,
Swindon, SN5 8RU, United KingdomMarketed by:
Cycle Pharmaceuticals Ltd
The Broers Building
21 JJ Thomson Ave
Cambridge, CB3 0FA, United KingdomTASCENSO ODT is a trademark of Handa Neuroscience, LLC.
Patented: see www.cyclepharma.com/product-patent-information
-
MEDICATION GUIDE
MEDICATION GUIDE
TASCENSO ODT® (tuh-SEN-soh ODT)
(fingolimod)
orally disintegrating tabletsThis Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 1/2025 Read this Medication Guide before you start taking TASCENSO ODT and each time you get a refill. There may be new information. If you are the parent of a child who is being treated with TASCENSO ODT, the following information applies to your child. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. What is the most important information I should know about TASCENSO ODT?
TASCENSO ODT may cause serious side effects, including:
1. Slow heart rate (bradycardia or bradyarrhythmia) when you start taking TASCENSO ODT. TASCENSO ODT can cause your heart rate to slow down, especially after you take your first dose. You will have a test, called an electrocardiogram (ECG), to check the electrical activity of your heart before you take your first dose of TASCENSO ODT.
All adults and children will be observed by a healthcare professional for at least 6 hours after taking their first dose of TASCENSO ODT. Children should also be observed by a healthcare professional for at least 6 hours after taking their first dose of 0.5 mg of TASCENSO ODT when switching from the 0.25 mg dose.
After you take your first dose of TASCENSO ODT, and after a child takes their first dose of 0.5 mg of TASCENSO ODT when switching from the 0.25 mg dose:
- Your pulse and blood pressure should be checked every hour.
- You should be observed by a healthcare professional to see if you have any serious side effects. If your heart rate slows down too much, you may have symptoms, such as:
- dizziness
- tiredness
- feeling like your heart is beating slowly or skipping beats
- chest pain
- If you have any symptoms of slow heart rate, they will usually happen during the first 6 hours after your first dose of TASCENSO ODT. Symptoms can happen up to 24 hours after you take your first dose of TASCENSO ODT.
- 6 hours after you take your first dose of TASCENSO ODT you will have another ECG. If your ECG shows any heart problems or if your heart rate is still too low or continues to decrease, you will continue to be observed.
- If you have any serious side effects after your first dose of TASCENSO ODT, especially those that require treatment with other medicines, you will stay in the medical facility to be observed overnight. You will also be observed for any serious side effects for at least 6 hours after you take your second dose of TASCENSO ODT the next day.
- If you have certain types of heart problems, or if you are taking certain types of medicines that can affect your heart, you will be observed overnight after you take your first dose of TASCENSO ODT.
Your slow heart rate will usually return to normal within 1 month after you start taking TASCENSO ODT. Call your healthcare provider or go to the nearest hospital emergency room right away if you have any symptoms of a slow heart rate.
If you miss 1 or more doses of TASCENSO ODT, you may need to be observed by a healthcare professional when you take your next dose. Call your healthcare provider if you miss a dose of TASCENSO ODT. See "How should I take TASCENSO ODT?"
2. Pregnancy. Please consult your healthcare provider before getting pregnant. You should avoid becoming pregnant while taking TASCENSO ODT or in the two months after you stop taking it because of the risk of harm to the baby.
3. Infections. TASCENSO ODT can increase your risk of serious infections that can be life-threatening and cause death. You should not receive live vaccines during treatment with TASCENSO ODT and for 2 months after you stop taking TASCENSO ODT. Talk to your healthcare provider before you receive a vaccine during treatment and for 2 months after treatment with TASCENSO ODT. If you receive a live vaccine, you may get the infection the vaccine was meant to prevent. Vaccines may not work as well when given during treatment with TASCENSO ODT.
Human Papilloma Virus (HPV). Due to risk of HPV infection please consult your healthcare provider for routine pap smear.
TASCENSO ODT lowers the number of white blood cells (lymphocytes) in your blood. This will usually go back to normal within 2 months of stopping treatment. Your healthcare provider may do a blood test to check your white blood cells before you start taking TASCENSO ODT. Call your healthcare provider right away if you have any of these symptoms of an infection during treatment with TASCENSO ODT, and for 2 months after your last dose of TASCENSO ODT:- fever
- tiredness
- body aches
- chills
- nausea
- vomiting
- headache with fever, neck stiffness, sensitivity to light, nausea, or confusion (these may be symptoms of meningitis, an infection of the lining around your brain and spine)
4. Progressive multifocal leukoencephalopathy (PML). PML is a rare brain infection that usually leads to death or severe disability. If PML happens, it usually happens in people with weakened immune systems but has happened in people who do not have weakened immune systems. Symptoms of PML get worse over days to weeks.
Call your healthcare provider right away if you have any new or worsening symptoms of PML, that have lasted several days, including:
- weakness on 1 side of your body
- loss of coordination in your arms and legs
- decreased strength
- problems with balance
- changes in your vision
- changes in your thinking or memory
- confusion
- changes in your personality
5. A problem with your vision called macular edema. Macular edema can cause some of the same vision symptoms as a multiple sclerosis (MS) attack (optic neuritis). You may not notice any symptoms with macular edema. If macular edema happens, it usually starts in the first 3 to 4 months after you start taking fingolimod, the active ingredient in TASCENSO ODT, but can happen at any time. Your healthcare provider should test your vision around the time you start taking TASCENSO ODT, 3 to 4 months after you start taking TASCENSO ODT, periodically while you continue taking TASCENSO ODT, and any time you notice vision changes during treatment with TASCENSO ODT. Your risk of macular edema is higher if you have diabetes or have had an inflammation of your eye called uveitis.
Call your healthcare provider right away if you have any of the following symptoms:
- blurriness or shadows in the center of your vision
- a blind spot in the center of your vision
- sensitivity to light
- unusually colored (tinted) vision
What is TASCENSO ODT?
TASCENSO ODT is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults and children 10 years of age and older.
It is not known if TASCENSO ODT is safe and effective in children under 10 years of age.
Who should not take TASCENSO ODT?
Do not take TASCENSO ODT if you:- have had a heart attack, unstable angina, stroke or mini-stroke (transient ischemic attack or TIA) or certain types of heart failure in the last 6 months.
- have certain types of irregular or abnormal heartbeat (arrhythmia), including patients in whom a heart finding called prolonged QT is seen on ECG before starting TASCENSO ODT.
- have a heart rhythm problem that needs treatment with certain medicines.
- are allergic to fingolimod or any of the ingredients in TASCENSO ODT. See the end of this leaflet for a complete list of ingredients in TASCENSO ODT. Symptoms of an allergic reaction may include rash, itchy hives, or swelling of the lips, tongue or face.
- taking other products containing fingolimod, the active ingredient in TASCENSO ODT.
Talk to your healthcare provider before taking TASCENSO ODT if you have any of these conditions, or do not know if you have any of these conditions.What should I tell my healthcare provider before taking TASCENSO ODT?
Before you take TASCENSO ODT, tell your healthcare provider about all your medical conditions, including if you had or now have:- an irregular or abnormal heartbeat (arrhythmia).
- a history of stroke or mini-stroke.
- heart problems, including heart attack or angina.
- a history of repeated fainting (syncope).
- a fever or infection, or you are unable to fight infections due to a disease or take or have taken medicines that lower your immune system.
- recently received a vaccine or are scheduled to receive a vaccine.
- chickenpox or have received the vaccine for chickenpox. Your healthcare provider may do a blood test for chickenpox virus. You may need to get the full course of the vaccine for chickenpox and then wait 1 month before you start taking TASCENSO ODT.
- your child has completed their vaccination schedule. Your child needs to have completed their vaccination schedule before starting treatment with TASCENSO ODT.
- eye problems, especially an inflammation of the eye called uveitis.
- diabetes.
- breathing problems, including during your sleep.
- liver problems.
- high blood pressure.
- any type of skin cancer, including basal cell carcinoma (BCC), melanoma or squamous cell carcinoma (SCC).
- are pregnant or plan to become pregnant. TASCENSO ODT may harm your unborn baby. Talk to your healthcare provider if you are pregnant or are planning to become pregnant. Tell your healthcare provider right away if you become pregnant while taking TASCENSO ODT or if you become pregnant within 2 months after you stop taking TASCENSO ODT.
- You should stop taking TASCENSO ODT 2 months before trying to become pregnant.
- If you are a female who can become pregnant, you should use effective birth control during your treatment with TASCENSO ODT and for at least 2 months after you stop taking TASCENSO ODT.
- are breastfeeding or plan to breastfeed. It is not known if TASCENSO ODT passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take TASCENSO ODT.
Tell your healthcare provider about all the medicines you take or have recently taken, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take medicines that affect your immune system, including corticosteroids, or have taken them in the past.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
Using TASCENSO ODT and other medicines together may affect each other causing serious side effects.
Do not take TASCENSO ODT with Gilenya or other medicines containing fingolimod.How should I take TASCENSO ODT?
- Adults and children will be observed by a healthcare professional for at least 6 hours after taking their first dose of fingolimod. Children should also be observed by a healthcare professional for at least 6 hours after taking their first dose of 0.5 mg of TASCENSO ODT when switching from the 0.25 mg dose. See "What is the most important information I should know about TASCENSO ODT?"
- Take TASCENSO ODT exactly as your healthcare provider tells you to take it.
- Take TASCENSO ODT 1 time each day.
- TASCENSO ODT may be taken with or without water.
- TASCENSO ODT may be taken with or without food.
- Store the TASCENSO ODT in the sealed blister pack until ready to take.
- Do not push the TASCENSO ODT through the foil.
- Use dry hands when opening the blister pack.
- Peel back the foil from 1 blister pack, then push the bottom of the blister pack to release the TASCENSO ODT and gently remove it.
- Take the orally disintegrating tablet (ODT) immediately after opening the blister pack.
- Place the ODT on the tongue and allow it to dissolve before swallowing.
- If you take too much TASCENSO ODT, call your healthcare provider or go to the nearest hospital emergency room right away.
- Do not stop taking TASCENSO ODT without talking with your healthcare provider first.
- Call your healthcare provider right away if you miss a dose of TASCENSO ODT. You may need to be observed by a healthcare professional for at least 6 hours when you take your next dose. If you need to be observed by a healthcare professional when you take your next dose of TASCENSO ODT you will have:
- an ECG before you take your dose
- hourly pulse and blood pressure measurements after you take the dose
- an ECG 6 hours after your dose
What are possible side effects of TASCENSO ODT?
TASCENSO ODT may cause serious side effects, including:- See "What is the most important information I should know about TASCENSO ODT?"
-
swelling and narrowing of the blood vessels in your brain. A condition called PRES (Posterior Reversible Encephalopathy Syndrome) has happened rarely in adults taking fingolimod, the active ingredient in TASCENSO ODT. Symptoms of PRES usually get better when you stop taking TASCENSO ODT.
However, if left untreated, it may lead to a stroke. Call your healthcare provider right away if you have any of the following symptoms:- sudden severe headache
- sudden confusion
- sudden loss of vision or other changes in your vision
- seizure
-
liver damage. TASCENSO ODT may cause liver damage. Your healthcare provider should do blood tests to check your liver before you start taking TASCENSO ODT and periodically during treatment. Call your healthcare provider right away if you have any of the following symptoms of liver damage:
- nausea
- vomiting
- stomach pain
- tiredness
- loss of appetite
- your skin or the whites of your eyes turn yellow
- dark urine
- breathing problems. Some people who take TASCENSO ODT have shortness of breath. Call your healthcare provider right away if you have new or worsening breathing problems.
- severe worsening of multiple sclerosis after stopping TASCENSO ODT. When TASCENSO ODT is stopped, symptoms of MS can return and become worse compared to before or during treatment. Many people who have worsening of MS symptoms after stopping TASCENSO ODT do not return to the level of function that they had before stopping TASCENSO ODT. This worsening happens most often within 12 weeks after stopping TASCENSO ODT but can happen later. Always talk to your healthcare provider before you stop taking TASCENSO ODT for any reason. Tell your healthcare provider if you have worsening symptoms of MS after stopping TASCENSO ODT.
- increased blood pressure. Your healthcare provider should check your blood pressure during treatment with TASCENSO ODT.
- types of skin cancer, including basal cell carcinoma, melanoma, and squamous cell carcinoma. Tell your healthcare provider if you have any changes in the appearance of your skin, including changes in a mole, a new darkened area on your skin, a sore that does not heal, or growths on your skin, such as a bump that may be shiny, pearly white, skin-colored, or pink. Your healthcare provider should check your skin for any changes at the start of and during treatment with TASCENSO ODT. Limit the amount of time you spend in sunlight and ultraviolet (UV) light. Wear protective clothing and use a sunscreen with a high sun protection factor.
- allergic reactions. Call your healthcare provider if you have symptoms of an allergic reaction, including a rash, itchy hives, or swelling of the lips, tongue or face.
- headache
- abnormal liver tests
- diarrhea
- cough
- flu
- inflammation of the sinuses (sinusitis)
- back pain
- stomach-area (abdominal) pain
- pain in arms or legs
These are not all of the possible side effects of TASCENSO ODT. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store TASCENSO ODT? - Store TASCENSO ODT in the blister pack in a dry place.
- Store TASCENSO ODT at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not open the blister pack until ready to take TASCENSO ODT.
- Keep TASCENSO ODT and all medicines out of the reach of children.
General information about the safe and effective use of TASCENSO ODT.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TASCENSO ODT for a condition for which it was not prescribed. Do not give TASCENSO ODT to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about TASCENSO ODT. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about TASCENSO ODT that is written for health professionals.What are the ingredients in TASCENSO ODT?
0.25 mg orally disintegrating tablets
Active ingredient: fingolimod lauryl sulfate.
Inactive ingredients: gelatin, mannitol, medium-chain triglycerides, sodium chloride, and sodium lauryl sulfate.
0.5 mg orally disintegrating tabletsActive ingredient: fingolimod lauryl sulfate.
Inactive ingredients: gelatin, mannitol, medium-chain triglycerides, sodium chloride, and sodium lauryl sulfate.
TASCENSO ODT is a trademark of Handa Neuroscience, LLC.
Manufactured by: Catalent Pharma Solutions, Swindon, United Kingdom
Marketed by: Cycle Pharmaceuticals Ltd, Cambridge, CB3 0FA, United Kingdom
For more information, go to www.tascenso.com or call +1 (888) 533-1625 -
PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Carton containing Blisters
Rx Only
FOR ORAL USE ONLY
NDC: 70709-062-30
CYCLE PHARMA
TASCENSO ODT® (fingolimod)
orally disintegrating tablets 0.25 mg
Contains 30 tablets
(3 blister cards of 10 tablets)PHARMACIST: Provide the accompanying
Medication Guide to each patient.
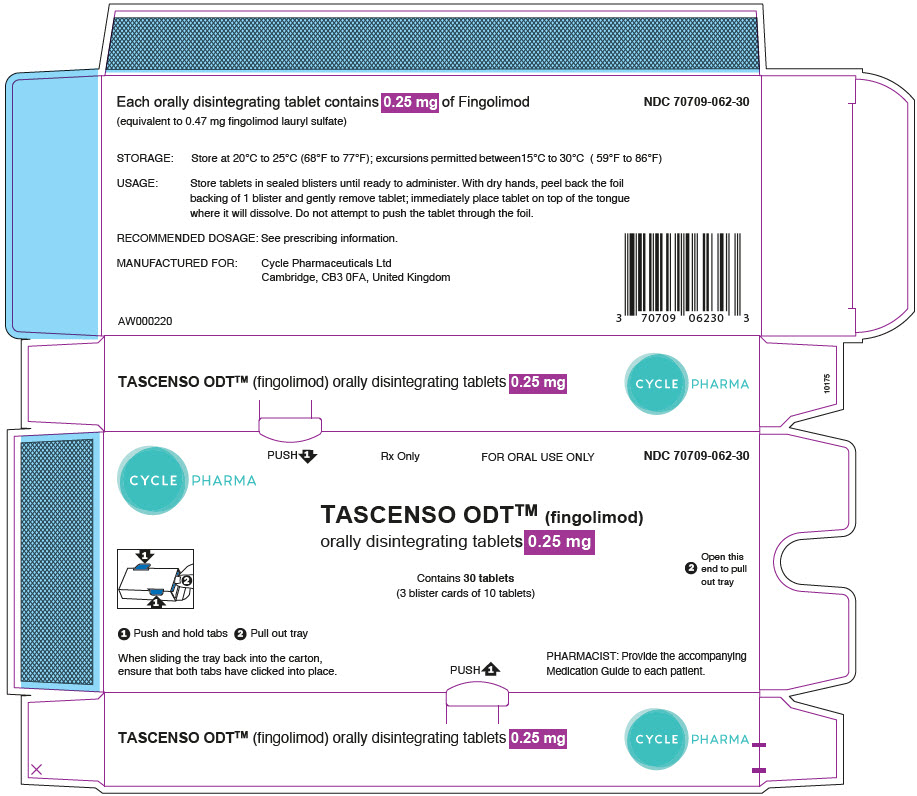
-
PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Carton containing Blisters
Rx Only
FOR ORAL USE ONLY
NDC: 70709-065-30
CYCLE PHARMA
TASCENSO ODT® (fingolimod)
orally disintegrating tablets 0.5 mg
Contains 30 tablets
(3 blister cards of 10 tablets)PHARMACIST: Provide the accompanying
Medication Guide to each patient.
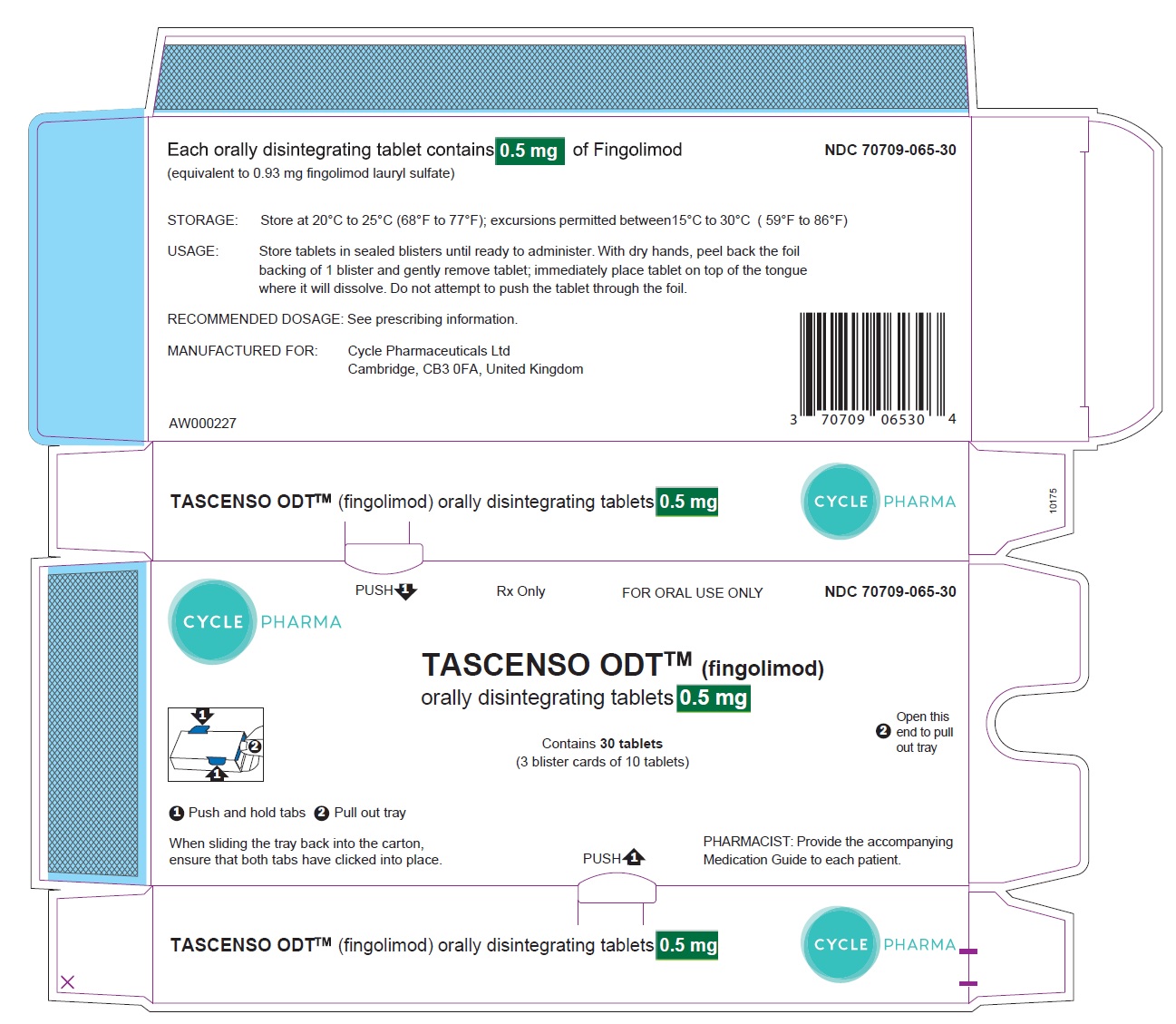
-
INGREDIENTS AND APPEARANCE
TASCENSO ODT
fingolimod lauryl sulfate tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70709-062 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fingolimod Lauryl Sulfate (UNII: J3HZQ9BE2S) (Fingolimod - UNII:3QN8BYN5QF) Fingolimod 0.25 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MANNITOL (UNII: 3OWL53L36A) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Sodium Chloride (UNII: 451W47IQ8X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND Size 13mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70709-062-30 3 in 1 CARTON 02/01/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214962 02/01/2023 TASCENSO ODT
fingolimod lauryl sulfate tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70709-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fingolimod Lauryl Sulfate (UNII: J3HZQ9BE2S) (Fingolimod - UNII:3QN8BYN5QF) Fingolimod 0.5 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MANNITOL (UNII: 3OWL53L36A) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Sodium Chloride (UNII: 451W47IQ8X) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND Size 16mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70709-065-30 3 in 1 CARTON 02/01/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214962 02/01/2023 Labeler - Cycle Pharmaceuticals Ltd (218215530) Registrant - Cycle Pharmaceuticals Ltd (218215530) Establishment Name Address ID/FEI Business Operations Catalent Pharma Solutions 237676320 MANUFACTURE(70709-062, 70709-065) , analysis(70709-062, 70709-065)
Trademark Results [TASCENSO ODT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TASCENSO ODT 90891701 not registered Live/Pending |
Handa Neuroscience, LLC 2021-08-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 .
. .
.