HAND SANITIZER WIPES by Zhejiang Qimei Cosmetics Co., Ltd. HAND SANITIZER WIPES
HAND SANITIZER WIPES by
Drug Labeling and Warnings
HAND SANITIZER WIPES by is a Otc medication manufactured, distributed, or labeled by Zhejiang Qimei Cosmetics Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
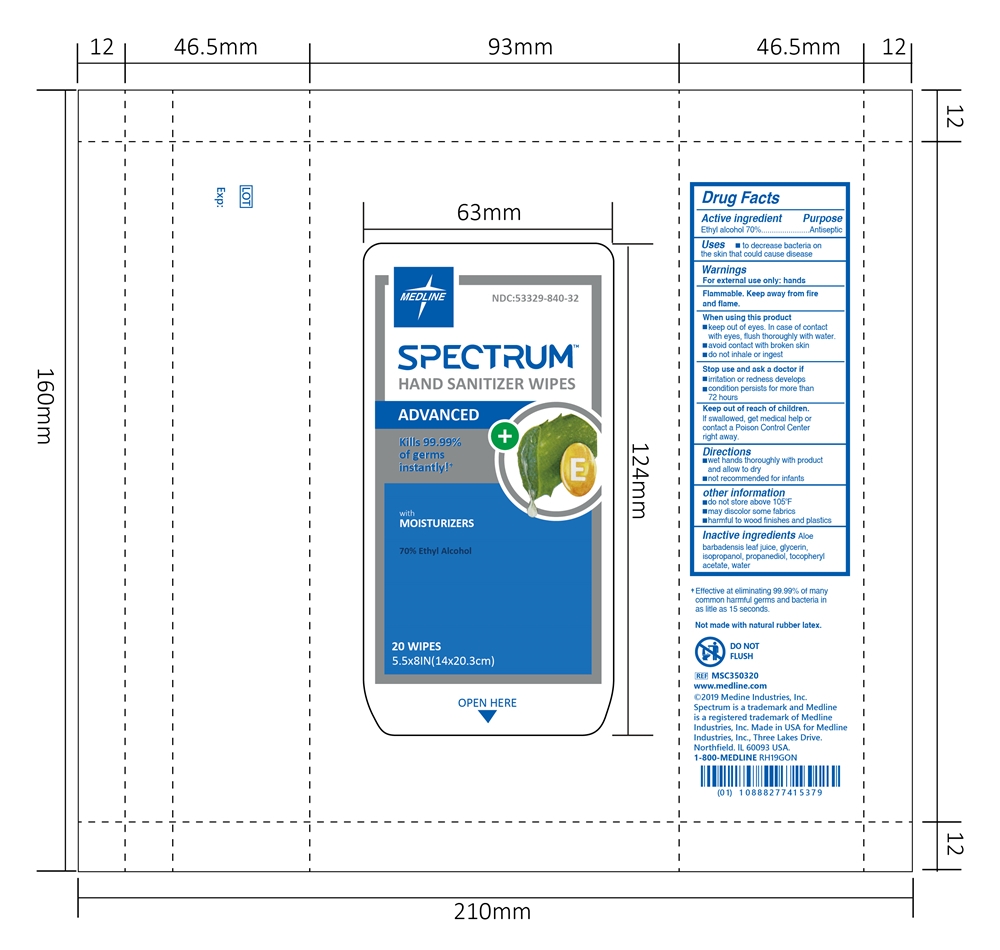
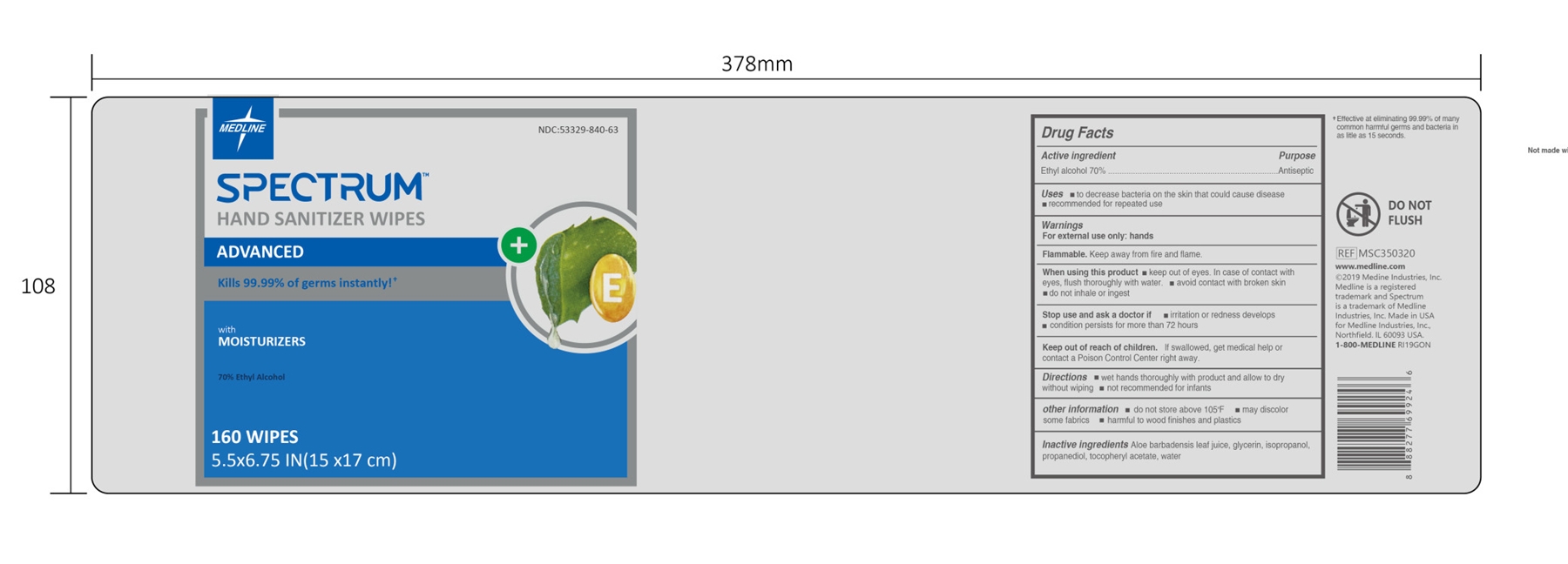
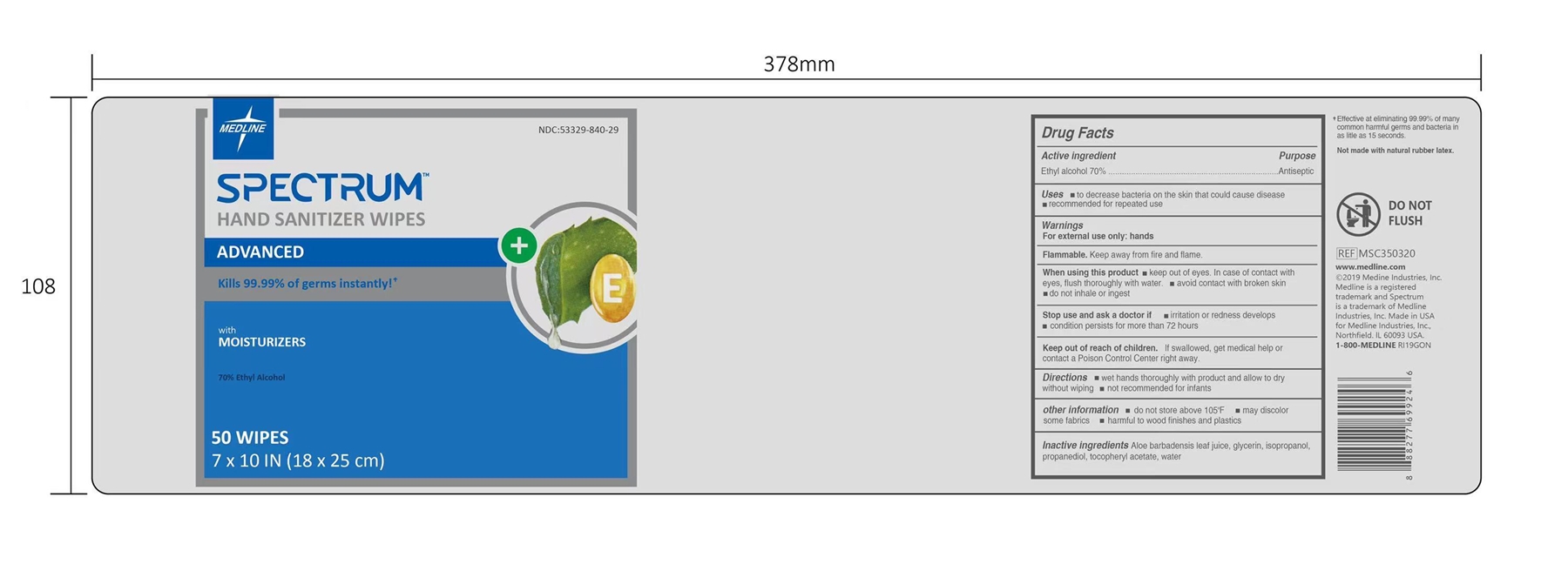
HAND SANITIZER WIPES- alcohol wipes cloth
Zhejiang Qimei Cosmetics Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HAND SANITIZER WIPES
When using this product
keep out of eyes. In case of contact with eyes, flush thoroughly with water.
avoid contact with broken skin
do not inhale or ingest
Stop use and ask a doctor if
irritation or redness develops
condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
other information
do not store above 105ºF
may discolor some fabrics
harmful to wood finishes and plastics
Inactive ingredients
Aloe barbadensis leaf juice, glycerin, isopropanol, propanediol, tocopheryl acetate, water
| HAND SANITIZER WIPES
alcohol wipes cloth |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Zhejiang Qimei Cosmetics Co., Ltd. (709887693) |
| Registrant - Zhejiang Qimei Cosmetics Co., Ltd. (709887693) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Qimei Cosmetics Co., Ltd. | 709887693 | manufacture(81773-001) | |