ZORBIUM- buprenorphine solution
Zorbium by
Drug Labeling and Warnings
Zorbium by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc., SpecGx LLC, Ivy Animal Health, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
HUMAN SAFETY WARNING
Abuse Potential
ZORBIUM contains buprenorphine, an opioid that exposes humans to risks of misuse, abuse, and addiction, which can lead to overdose and death. Use of buprenorphine may lead to physical dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of ZORBIUM. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drugs or alcohol) or mental illness (e.g. depression).Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with accidental exposure to or with misuse or abuse of ZORBIUM. Monitor for respiratory depression if human exposure to buprenorphine occurs. Misuse or abuse of buprenorphine by swallowing, snorting, or injecting poses a significant risk of overdose and death.Accidental Exposure
Because of the potential for adverse reactions associated with accidental exposure, ZORBIUM should only be administered by veterinarians or veterinary technicians who are trained in the handling of potent opioids. Accidental exposure to even one tube of ZORBIUM, especially in children, can result in a fatal overdose of buprenorphine.Risks From Concurrent Misuse or Abuse with Benzodiazepines or Other CNS Depressants Concurrent misuse or abuse of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
See Human Safety Warnings for detailed information.
-
DESCRIPTION:
ZORBIUM is a volatile liquid transdermal solution intended for topical application that provides continuous, systemic delivery of the opioid analgesic, buprenorphine. Buprenorphine belongs to the opioid class of drugs and is a narcotic under the Controlled Substances Act due to its chemical derivation from thebaine. Once ZORBIUM is applied to the skin, rapid drying results in dermal absorption and sequestration of buprenorphine into the stratum corneum. Each mL of ZORBIUM contains 20 mg buprenorphine (administered as 21.56 mg of buprenorphine hydrochloride). The inactive ingredients are dehydrated alcohol, padimate O, butylated hydroxyanisole, and butylated hydroxytoluene.
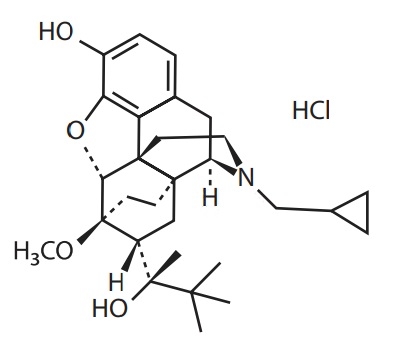
Buprenorphine hydrochloride has an empirical formula of C29H41NO4 HCl and a molecular weight of 504.10.
The chemical structure of buprenorphine HCl is: - INDICATION:
-
DOSAGE AND ADMINISTRATION:
This product should only be administered by veterinary personnel.
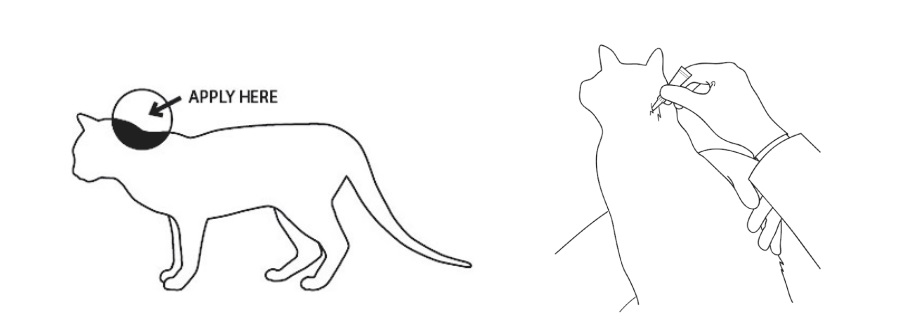
ZORBIUM is for administration only once for the surgical procedure. ZORBIUM should be applied 1 to 2 hours before surgery. A single application provides analgesia for 4 days. ZORBIUM should only be applied topically to the dorsal cervical area at the base of the skull. Do not apply if dorsal cervical skin is diseased or injured. The dosage of ZORBIUM is 1.2 – 3.1 mg/lb (2.7 – 6.7 mg/kg) administered topically as the entire tube contents according to the following dosing table:
Pounds of Body Weight
Kilograms of Body Weight
Dose of ZORBIUM
2.6 to 6.6
1.2 to 3
0.4 mL (8 mg) pink tube
>6.6 to 16.5
>3 to 7.5
1 mL (20 mg) green tube
Dose Application
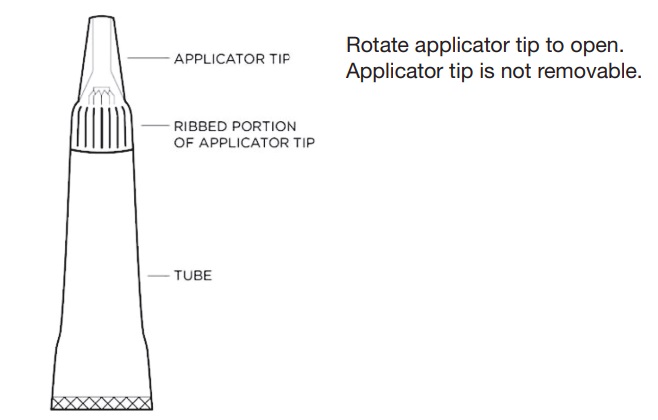
Wear impermeable latex or nitrile gloves, protective glasses, and a laboratory coat to prevent direct solution contact with human skin, eyes, or mucosa when handling and applying the solution. Do not dispense ZORBIUM for administration at home by the pet owner (see HUMAN SAFETY WARNINGS).Figure 1 - Diagram of tube components.
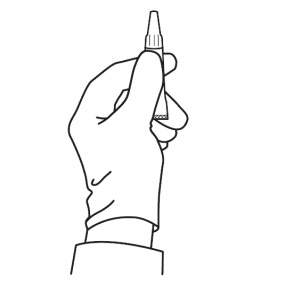
Figure 2 – Proper grasp of the applicator tube: To prepare to open the tube for application, the tube must be held in an upright position to avoid spilling contents. Grasp the tube just beneath the ribbed portion of the tip.
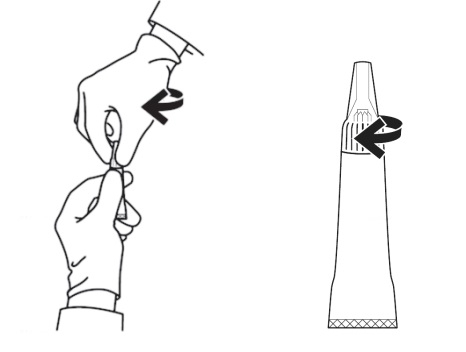
Figure 3 – Opening the applicator tube: Keeping the tube upright, grasp the ribbed portion of the tip, and turn the applicator tip in either direction at least 180° to open the tube. The applicator tip is designed to stay on the tube and should not be removed. The tube is ready for application when a breaking of the seal is felt.
Figure 4 – Solution application: Fur should not be clipped. Do not apply to injured or diseased skin. Gently hold the cat both before and after application to prevent the cat from shaking or rubbing.
Part the fur and apply the tip of the tube directly onto the skin at the base of the head. Squeeze the tube 2 – 3 times to empty the contents without moving the tube or the tip. Lift the tube directly away from the skin, avoiding contact of the tip with the cat’s fur.
- CONTRAINDICATIONS:
-
WARNINGS:
HUMAN SAFETY WARNINGS:
Not for use in humans. Keep this and all medications out of reach of children and pets.Human User Safety While Handling ZORBIUM in the Hospital:
Protective Covering: Do not come into direct contact with ZORBIUM. Wear impermeable latex or nitrile gloves, protective glasses, and a laboratory coat when applying ZORBIUM.Mucous Membrane or Eye Contact During Application:
Direct contact of ZORBIUM with the eyes, oral, or other mucous membranes could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral, or other mucous membrane contact is made during application, flush the area with water and contact a physician immediately. If wearing contact lenses, flush the eye first and then remove the contact lens.Skin Contact During Application:
Following application to the cat, allow a minimum drying time of 30 minutes before direct contact with the application site. If human skin is accidentally exposed to ZORBIUM, wash the exposed area immediately with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.Drug Abuse, Addiction, and Diversion of Opioids:
Controlled Substance:
ZORBIUM contains buprenorphine, a Schedule III controlled substance with an abuse potential similar to other Schedule III opioids.Abuse:
ZORBIUM contains buprenorphine, an opioid substance, that can be abused and is subject to misuse, abuse, and addiction, which may lead to overdose and death. This risk is increased with concurrent use of alcohol and other central nervous system depressants, including other opioids and benzodiazepines.ZORBIUM should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Prescription drug abuse is the intentional, non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse.
Storage and Disposal:
ZORBIUM is a Schedule III opioid. Store in a locked cabinet according to federal and state controlled substance requirements/guidelines. Any unused or expired tubes must be destroyed by a reverse distributor; for further information, contact your local DEA field office or call Elanco US Inc. at 1-888-545-5973.Information for Physician:
ZORBIUM transdermal solution contains a mu opioid partial agonist (20 mg buprenorphine/mL). In the case of an emergency, provide the physician with this package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant.ANIMAL SAFETY WARNINGS:
For topical use in cats only. This product should only be administered by veterinary personnel.
Do not apply ZORBIUM if the application site at the dorsal cervical area has diseased or injured skin.
Do not apply ZORBIUM to anatomic areas other than the dorsal cervical area because absorption characteristics may be different. -
PRECAUTIONS:
Following anesthesia and opioid analgesia, body temperature should be monitored postoperatively for immediate hypothermia and subsequent hyperthermia. Hyperthermia can occur and persist after the hypothermic effects of anesthesia have resolved.
The safe use of ZORBIUM has not been evaluated in debilitated cats or cats with renal, hepatic, cardiac, or respiratory disease.
The safe use of ZORBIUM has not been evaluated in cats that are pregnant, lactating, or intended for breeding.
The safe use of ZORBIUM has not been evaluated in cats younger than four months old.
The safe use of ZORBIUM has not been evaluated in cats weighing less than 2.6 pounds or more than 16.5 pounds.
-
ADVERSE REACTIONS:
In a randomized, multi-centered, double-masked, field study, ZORBIUM™ (buprenorphine transdermal solution) (N=113) or vehicle control (N=109) was administered to cats prior to elective surgical reproductive sterilization (castration/ovariohysterectomy) in conjunction with forelimb onychectomy. Cats enrolled in the study were 4 months to 5 years of age and weighed 1.1 to 5.7 kg (2.5 to 12.5 lb). Clinical observations and physiological parameters were monitored prior to, during, and after surgery for 96 hours after sternal recumbency.
Supplemental heat and fluids were allowed. There were no deaths during the study and no cats received an opioid reversal agent. Three ZORBIUM and 2 vehicle control cats were removed due to hyperthermia suspected to be treatment related. One ZORBIUM cat was removed due to fractious behavior 30 minutes following surgery. Adverse reactions were defined as any single excursion outside the normal range, as defined: 100.5 – 102.5 ˚F body temperature; 60 – 120 mmHg mean arterial pressure; 88 – 180 beats per minute for heart rate; 24 – 44 breaths per minute for respiratory rate. A summary of adverse reactions during anesthesia (from anesthetic induction through recovery defined as sternal recumbency) is provided in Table 1.Table 1. Adverse Reactions During Anesthesia: Adverse Reaction*
ZORBIUM
(N=113)Vehicle Control
(N=109)Hypothermia
37 (32.7%)
29 (26.6%)
Hypotension
31 (27.4%)
28 (25.7%)
Hypertension
27 (23.9%)
18 (16.5%)
Tachycardia
14 (12.4%)
14 (12.8%)
Sedation
12 (10.6%)
7 (6.4%)
Oxygen saturation ≤ 90%
6 (5.3%)
2 (1.8%)
Bradycardia
4 (3.5%)
2 (1.8%)
Hyperthermia
3 (2.7%)
4 (3.7%)
*Physiological adverse reactions were defined as any single excursion outside the normal range at any 10 minute interval during the entire duration of anesthesia.
After recovery, cats were observed in the hospital and underwent physiological assessments that included indirect mean arterial blood pressure, heart rate, respiratory rate, body temperature, lung auscultation, heart auscultation, and assessments of urination, defecation, and appetite. A summary of adverse reactions after anesthetic recovery (sternal recumbency) in all cats is reported in Table 2.
Table 2. Adverse Reactions After Anesthetic Recovery: Adverse Reaction*
ZORBIUM
(N=113)Vehicle Control
(N=109)Hypothermia
107 (94.7%)
105 (96.3%)
Hyperthermia
84 (74.3%)
62 (56.9%)
Sedation
64 (56.6%)
48 (44.0%)
Tachypnea
56 (49.6%)
70 (64.2%)
Hypotension
50 (44.2%)
51 (46.8%)
Hypertension
42 (37.2%)
34 (31.2%)
Bradycardia
34 (30.1%)
45 (41.3%)
Tachycardia
32 (28.3%)
39 (35.8%)
Anorexia
25 (22.1%)
32 (29.4%)
Dysphoria
20 (17.7%)
29 (26.6%)
Diarrhea
11 (9.7%)
11 (10.1%)
Bradypnea
11 (9.7%)
7 (6.4%)
Leukocytosis
6 (5.3%)
4 (3.7%)
Hyperactivity
2 (1.8%)
9 (8.3%)
*Physiological adverse reactions were defined as any single excursion outside the normal range following anesthetic recovery (sternal recumbency) through 4 days postoperatively.
Hyperthermia was the only adverse event observed in more than 10% of cats in the ZORBIUM group after the day of surgery (24 – 96 hours). The percentage of cats in the ZORBIUM group with hyperthermia decreased over time from 66.4% on Day 0 to 28.3% on Day 1, and to 6.2% by Day 4. A summary of adverse reactions (from anesthetic recovery through 96 hours after recovery) in cats in the ZORBIUM group by study day is reported in Table 3.
Table 3. Adverse Reactions in ZORBIUM Cats (N=113) by Day: Adverse Reaction*
Day 0
Day 1
Day 2
Day 3
Day 4
Hypothermia
106 (93.8%)
2 (1.8%)
2 (1.8%)
2 (1.8%)
2 (1.8%)
Hyperthermia
75 (66.4%)
32 (28.3%)
18 (15.9%)
14 (12.4%)
7 (6.2%)
Sedation
64 (56.6%)
0 (0.0%)
0 (0.0%)
0 (0.0%)
0 (0.0%)
Tachypnea
51 (45.1%)
5 (4.4%)
2 (1.8%)
3 (2.7%)
4 (3.5%)
Hypotension
42 (37.2%)
2 (1.8%)
1 (0.9%)
4 (3.5%)
2 (1.8%)
Hypertension
28 (24.8%)
2 (1.8%)
1 (0.9%)
1 (0.9%)
1 (0.9%)
Anorexia
25 (22.1%)
3 (2.7%)
1 (0.9%)
0 (0.0%)
0 (0.0%)
Bradycardia
24 (21.2%)
3 (2.7%)
2 (1.8%)
3 (2.7%)
5 (4.4%)
Tachycardia
24 (21.2%)
4 (3.5%)
0 (0.0%)
1 (0.9%)
1 (0.9%)
Dysphoria
20 (17.7%)
0 (0.0%)
0 (0.0%)
0 (0.0%)
0 (0.0%)
Bradypnea
8 (7.1%)
2 (1.8%)
0 (0.0%)
0 (0.0%)
1 (0.9%)
Hyperactivity
1 (0.9%)
0 (0.0%)
0 (0.0%)
0 (0.0%)
1 (0.9%)
*Physiological adverse reactions were defined as any single excursion outside the normal range following anesthetic recovery (sternal recumbency) through 96 hours postoperatively.
-
CONTACT INFORMATION:
To report suspected adverse events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US Inc. at 1-888-545-5973.
For additional information about reporting adverse drug experience for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY:
Mechanism of Action: Buprenorphine exerts its analgesic effect via high affinity binding to various subclasses of opiate receptors, particularly mu, in the central nervous system. Buprenorphine analgesic and adverse reactions are mediated by mu opioid receptor agonism. Due to its partial agonist activity, buprenorphine exhibits a ceiling effect to its actions and thus has a greater therapeutic index compared to full mu opioid receptor agonists such as morphine. Buprenorphine binds tightly to and disassociates slowly from the opioid receptor. Therefore, the pharmacological effects of buprenorphine are not directly related to plasma concentrations.
Pharmacokinetics: Following application of ZORBIUM, solvent evaporation coupled with the permeation enhancer action results in rapid absorption and sequestration of the buprenorphine into the skin. ZORBIUM provides analgesia within 1 – 2 hours following administration and continually releases buprenorphine from the skin into the systemic circulation over a period of days. The mean (range) time to reach peak concentration (tmax) was 7.33 (1 – 24) hours. Due to buprenorphine elimination being faster than absorption from the skin, ZORBIUM exhibits flip-flop pharmacokinetics where the absorption determines its terminal half-life (mean 64.9 hours [range 39.1 – 85.7 hours]). The estimated absolute bioavailability (F) of transdermal administration was in the order of 16% [90% confidence interval (CI) =11.8% – 21.7%].
Buprenorphine is extensively metabolized by the liver in humans, the primary route being N-dealkylation to norbuprenorphine by cytochrome P450 enzymes. Both buprenorphine and norbuprenorphine form inactive glucuronide conjugates and are excreted by the bile into the gastrointestinal tract. The cat is devoid of uridine diphosphate glucuronosyltransferase enzymes (UGT1A6 and UGT1A9) responsible for conjugation and therefore conjugated metabolites may be absent. Norbuprenorphine is considered an active metabolite of buprenorphine, though it has one-fiftieth the analgesic activity of buprenorphine in rats. Buprenorphine extensively binds (95 – 98%) to plasma proteins.
-
EFFECTIVENESS:
The effectiveness of ZORBIUM was demonstrated in cats that underwent elective reproductive sterilization in conjunction with forelimb onychectomy surgery in a randomized, multi-centered, double-masked, vehicle-controlled, field study across 12 investigative sites. Enrolled cats were between 4 months to 5 years of age and weighed 2.5 to 12.5 pounds (1.1 – 5.7 kg).
Cats in the ZORBIUM group received a single dose of 8 mg or 20 mg of buprenorphine according to body weight (see DOSAGE AND ADMINISTRATION). Cats in the vehicle control group received a transdermal solution of 50 mg/mL padimate O in ethanol. The dose was administered topically onto the dorsal cervical skin 1 – 2 hours prior to anesthetic induction for surgery. For intraoperative analgesia, all cats in the study received a single intramuscular injection of an alpha2-agonist 30 minutes prior to anesthetic induction, and a 4-point metacarpal ring block with lidocaine after induction. The adequacy of pain control was scored through 96 hours after surgery. If pain control was considered inadequate at any time following treatment, rescue analgesia was provided immediately. Treatment success was defined as a cat that did not require rescue analgesia, need opioid reversal, or experience an adverse event suspected to be related to treatment through the entire 96-hour post-recovery period. A cat was considered a treatment failure if it had inadequate pain control, required opioid reversal, or experienced an adverse event suspected to be related to treatment.
A total of 19 ZORBIUM and 63 vehicle control cats were removed from the study due to inadequate pain control. Most of these failures occurred on the day of surgery in both groups; however, there were 4 ZORBIUM group cats and 5 vehicle control cats removed due to inadequate pain control between 1 and 3 days after surgery.
Effectiveness was evaluated in 219 cats (112 in the ZORBIUM group and 107 cats in the vehicle control group), and field safety was evaluated in 222 cats (113 cats in the ZORBIUM group and 109 cats in the vehicle control group). Of the 112 cats in the ZORBIUM group, 89 were treatment successes; of the 107 vehicle control cats, 42 were treatment successes. Comparison of the ZORBIUM group and the vehicle control group demonstrated a statistically significant difference in the treatment success rates (p = 0.0003).
Hypothermia was common in both groups during surgery. The overall mean postoperative body temperature was higher in the ZORBIUM group than in the vehicle control group. Mean postoperative body temperatures in the ZORBIUM group were above the normal range at 4 and 8 hours postoperatively (mean±SD of 102.7°±1.2 °F and 102.6°±1.0 °F, respectively [normal range: 100.5 – 102.5 °F]). Mean indirect arterial blood pressure (MAP) was similar between the 2 treatment groups over time. Urination, defecation, appetite, and daily body weights after surgery were not affected by ZORBIUM administration. Fifteen cats in the ZORBIUM group had an increased fibrinogen at discharge, compared to 2 cats in the vehicle control group.
Fluid administration (intravenous and subcutaneous) and supplemental heat support after surgery were the most common concurrent treatments and were used similarly in both groups.
-
TARGET ANIMAL SAFETY:
Twelve Day Target Animal Safety Study: In a 12-day laboratory study, ZORBIUM was administered to 32 healthy four-month-old domestic cats (8 cats per group) at 0 mg/kg (vehicle control), 6.7 mg/kg (1X), 13.3 mg/kg (2X), and 20 mg/kg (3X) as a topical application to the dorsal cervical area every 4 days for a total of 3 doses. Dose-independent euphoria, mild dysphoria, and mydriasis were observed after ZORBIUM administration. Maximum scores (for euphoria, dysphoria, and mydriasis) in the ZORBIUM groups reached 3 (mildly dysphoric) between 1 – 2 hours after the first dose. On the other 2 dosing days (Days 4 and 8), maximum scores were 2 (euphoric). Euphoria in some cats persisted from 36 to 72 hours.
On dosing day 1, mydriasis was observed in approximately half the cats administered ZORBIUM by 24 hours after dosing (peaked in all ZORBIUM groups at 8 hours) and was not observed between 48 – 93 hours. After the day 8 dose (third dose), it was rarely observed (1 cat in 1X group; 2 cats in 3X at 48 hours; 1 cat in 2X at 72 hours).
Cats administered ZORBIUM had higher body temperatures compared to the vehicle control group throughout the study. Following the initial dose, the mean temperatures in cats administered ZORBIUM increased above normal and were up to 1.8 ºF greater than the vehicle control group. Increased body temperature primarily occurred during the first 8 hours after the initial dose and was observed in the majority of cats administered ZORBIUM. Elevated temperatures ranged from 102.6 °F to 104.5 °F. The highest temperatures occurred at 2 hours after the first dose, gradually decreasing by 24 hours. By 3 days after dose administration, body temperatures in cats administered ZORBIUM had returned to levels observed in the vehicle control group. After the second and third doses (days 4 and 8), mean temperatures in all ZORBIUM groups were again higher than in the vehicle control group, but not higher than the normal reference range.
Constipation was recorded for 20 cats (1 vehicle control; 3 in 1X; 4 in 2X; 6 in 3X groups) after the first dose. The constipation was mild and transient. Three cats (2 in the 1X group and 1 in the 3X group) were administered a laxative. ZORBIUM had no clinically significant effects on heart rate or respiratory rate. There were no clinically relevant changes to serum chemistry, hematology, or urinalysis outcomes. Histopathology evaluations revealed mild inflammation of skin at the application site.
Seven Day Target Animal Safety Study: In a 7-day laboratory study, ZORBIUM was administered once topically to the dorsal cervical area of 24 healthy adult domestic cats (6 cats/group) at 0 mg/kg (0X; vehicle control), 3.3 (0.5X), 10 (1.5X), or 30 mg/kg (4.5X the maximal dose of 6.7 mg/kg). Cats were observed for 7 days after the single dose. Clinical results were similar to the 12-day margin of safety study, even at the higher dose of 30 mg/kg, except for transient increases in heart rate in the ZORBIUM groups compared to the vehicle control group. Mean heart rates in ZORBIUM groups were higher than in the vehicle control group from 2 hours through approximately 48 hours after dose administration. Tachycardia (>240 beats per minute) occurred in two cats in the 0.5X group and two cats in the 4.5X group for at least one timepoint after dose administration. The highest heart rate was 260 (in the 0.5X group) and no dose relationship was evident. Dose-independent euphoria, mild dysphoria, and mydriasis were noted in the ZORBIUM groups. Mild constipation and/or abdominal distension were observed with ZORBIUM administration. Transient increases in plasma chloride and sodium concentrations in the ZORBIUM groups compared to vehicle control group indicated mild dehydration.
Cardiovascular Safety Study: In a 12-day cardiovascular laboratory safety study, ZORBIUM was administered to 8 healthy adult cats (4 cats per group) at 0 mg/kg (vehicle control) and 6.7 mg/kg (1X) as a topical application to the dorsal cervical region every 4 days for a total of 3 doses. Continuous, direct physiological monitoring (telemetry) was conducted from 2 hours prior to the first dose through 4 days following the third (final) dose. Body temperature increases averaged <0.4 oF in ZORBIUM group cats over vehicle control cats. In the vehicle control group, 102.6 °F was the maximum temperature, observed at 93 hours after the first dose. In the ZORBIUM group, 103.4 °F was the maximum temperature, observed at 20 hours after the first dose. Heart rate (HR) increased an average of 15.2 beats/minute in the ZORBIUM group cats compared to vehicle control cats. The maximum heart rate in the ZORBIUM group reached 231 beats/minute. In the vehicle control group, the maximum heart rate was 219 beats/minute. Blood pressure (arterial systolic, diastolic, and mean) in the ZORBIUM group cats was not significantly different from the vehicle control cats. There were no clinically significant effects of ZORBIUM on qualitative electrocardiogram results.
- STORAGE INFORMATION:
-
HOW SUPPLIED:
ZORBIUM is available in applicator tubes that deliver a dose volume of 0.4 mL or 1 mL (20 mg/mL buprenorphine) in multi-packs of 10 tubes.
Approved by FDA under NADA # 141-547
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140 USAZORBIUM, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2021 Elanco or its affiliatesRev. Date 02/2022
PA102712X
ElancoTM -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
ElancoTM
ZorbiumTM
(buprenorphine transdermal solution)
20 mg/mL
For topical application in cats only
Opioid analgesic
2.6 to 6.6 lb
cats0.4 mL
10 single dose 0.4 mL tubes
Approved by FDA under NADA # 141-547
Caution: Federal law restricts this drug to
use by or on the order of a licensed
veterinarian.HUMAN SAFETY WARNING:
Due to serious human safety
and abuse concerns read the
entire product insert before
using this drug, including the
complete Boxed Warning.Indication: ZORBIUM is indicated for the
control of postoperative pain associated with
surgical procedures in cats. -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
ElancoTM
ZorbiumTM
(buprenorphine transdermal solution)
20 mg/mL
For topical application in cats only
Opioid analgesic
>6.6 to 16.5 lb
cats1 mL
10 single dose 1 mL tubes
Approved by FDA under NADA # 141-547
Caution: Federal law restricts this drug to
use by or on the order of a licensed
veterinarian.HUMAN SAFETY WARNING:
Due to serious human safety
and abuse concerns read the
entire product insert before
using this drug, including the
complete Boxed Warning.Indication: ZORBIUM is indicated for the
control of postoperative pain associated with
surgical procedures in cats. -
INGREDIENTS AND APPEARANCE
ZORBIUM
buprenorphine solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-0078 Route of Administration TRANSDERMAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE (UNII: 40D3SCR4GZ) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-0078-1 10 in 1 CARTON 1 0.4 mL in 1 TUBE 2 NDC: 58198-0078-2 10 in 1 CARTON 2 1 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141547 01/20/2022 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations SpecGx LLC 163205300 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Ivy Animal Health, Inc. 080698078 MANUFACTURE, PACK, LABEL
Trademark Results [Zorbium]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZORBIUM 88824689 not registered Live/Pending |
ELANCO US INC. 2020-03-06 |
 ZORBIUM 87080379 not registered Live/Pending |
ELANCO US INC. 2016-06-22 |
 ZORBIUM 85676599 not registered Dead/Abandoned |
Eli Lilly and Company 2012-07-13 |
 ZORBIUM 78070333 2851805 Live/Registered |
INTELLECTUAL PROPERTY HOLDINGS, LLC 2001-06-21 |
 ZORBIUM 77660375 not registered Dead/Abandoned |
Eli Lilly and Company 2009-01-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.