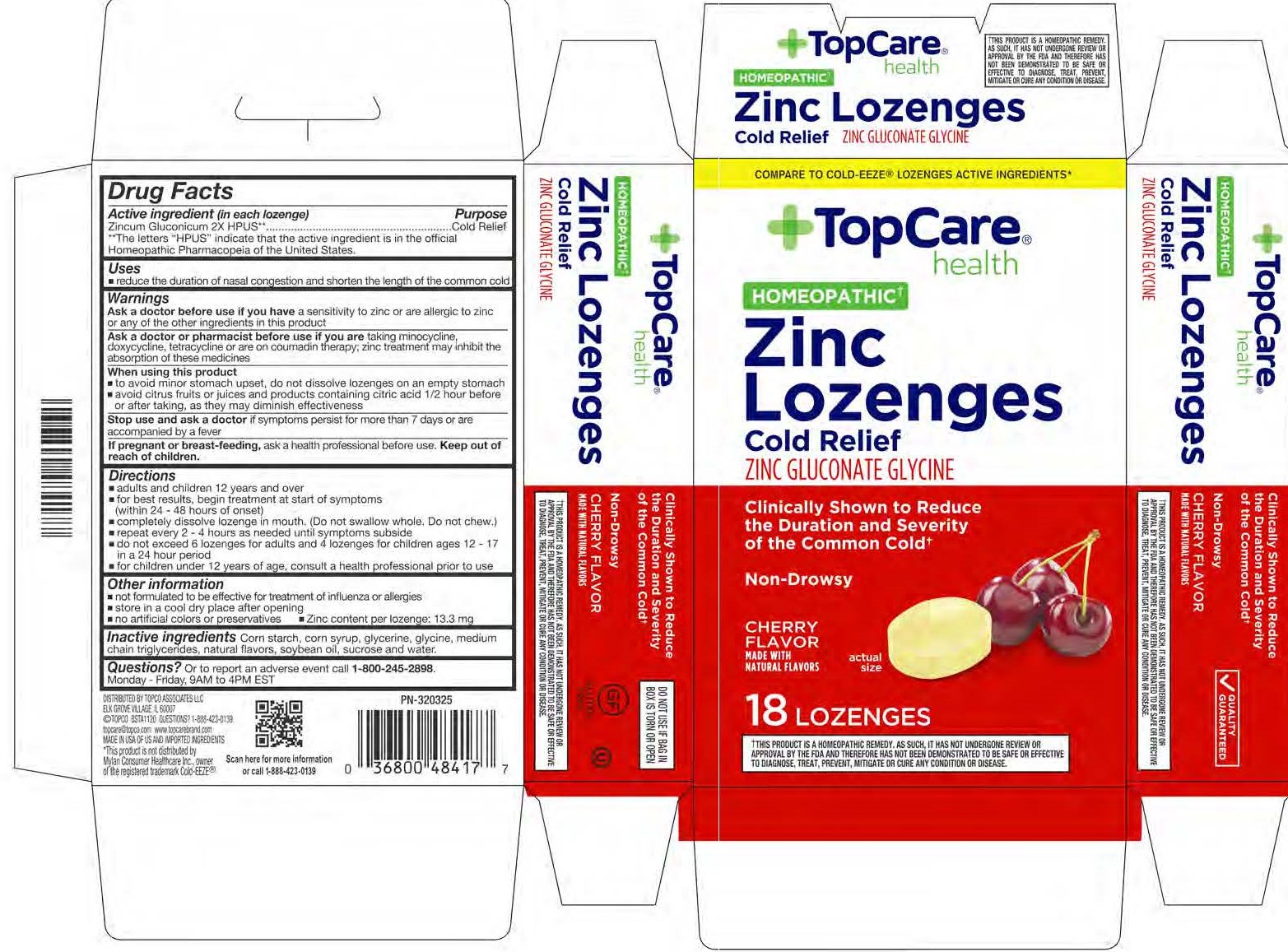
TopCare Cherry Zinc Lozenges by Topco / Bestco LLC Zincum Gluconicum 2X
TopCare Cherry Zinc Lozenges by
Drug Labeling and Warnings
TopCare Cherry Zinc Lozenges by is a Homeopathic medication manufactured, distributed, or labeled by Topco, Bestco LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TOPCARE CHERRY ZINC LOZENGES- zinc gluconate lozenge
Topco
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Zincum Gluconicum 2X
Active ingredient (in each lozenge)
Zincum Gluconicum 2X HPUS**
**The letters "HPUS" indicate that the active ingredient is in the official Homeopathic Pharmacopeia of the United States.
Warnings
Ask a doctor before use if you have a sensitivity to zinc or are allergic to zinc or any of the ingredients in this product.
Ask a doctor or pharmacist before use if you are taking minocycline, doxycycline, tetracycline or are on coumadin therapy; zinc treatment may inhibit the absorption of these medicines.
When using this product
- to avoid minor stomach upset, do not dissolve lozenges on an empty stomach
- avoid citrus fruits or juices and products containing citric acid 1/2 hour before or after taking, as they may diminish effectiveness
Directions
- adults and children 12 years and over
- for best results, begin treatment at start of symptoms (within 24 - 48 hours of onset)
- completely dissolve lozenge in mouth. (Do not swallow whole. Do not chew.)
- repeat every 2 - 4 hours as needed until symptoms subside
- do not exceed 6 lozenges for adults and 4 lozenges for children ages 12 - 17 in a 24 hour period
- for children under 12 years of age, consult a health professional prior to use
Other information
- not formulated to be effective for treatment of influenza or allergies
- store in a cool dry place after opening
- no artificial colors or preservatives
- Zinc content per lozenge: 13.3 mg
| TOPCARE CHERRY ZINC LOZENGES
zinc gluconate lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Topco (006935977) |
| Registrant - Bestco LLC (002149136) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bestco LLC | 002149136 | manufacture(76162-401) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.