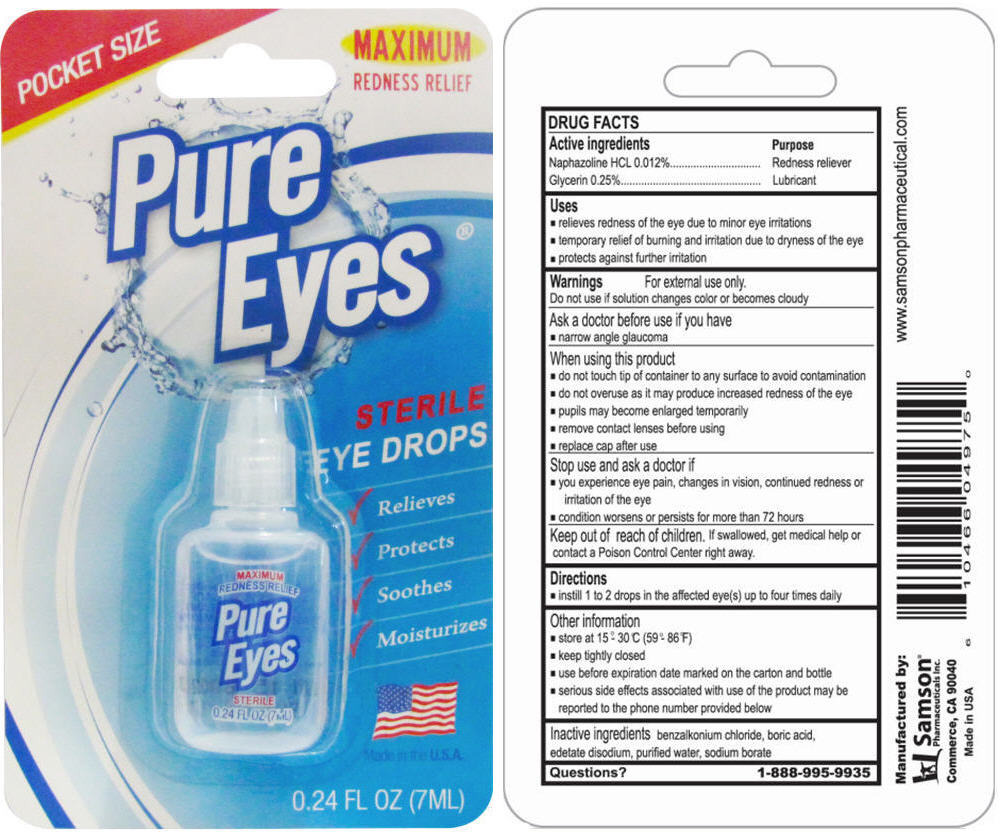
PURE EYES STERILE EYES- naphazoline hydrochloride and glycerin solution/ drops
Pure Eyes Sterile Eyes by
Drug Labeling and Warnings
Pure Eyes Sterile Eyes by is a Otc medication manufactured, distributed, or labeled by SAMSON PHARMACEUTICAL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
When using this product
- ♦ Do not touch tip of container to any surface to avoid contamination
- ♦ Do not overuse as it may products increased redness of the eye
- ♦ Pupils may be come enlarged temporarily
- ♦ Remove contact lenses before using
- ♦ Replace cap after use
- Directions
- Other information
- Inactive ingredients
- Question?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 7 mL Bottle Blister Pack
-
INGREDIENTS AND APPEARANCE
PURE EYES STERILE EYES
naphazoline hydrochloride and glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 20146-7000 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Naphazoline Hydrochloride (UNII: MZ1131787D) (Naphazoline - UNII:H231GF11BV) Naphazoline Hydrochloride 0.1 mg in 1 mL Glycerin (UNII: PDC6A3C0OX) (Glycerin - UNII:PDC6A3C0OX) Glycerin 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) boric acid (UNII: R57ZHV85D4) edetate disodium (UNII: 7FLD91C86K) water (UNII: 059QF0KO0R) sodium borate (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 20146-7000-1 1 in 1 BLISTER PACK 01/01/2015 1 7 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part349 01/01/2015 Labeler - SAMSON PHARMACEUTICAL (088169581) Establishment Name Address ID/FEI Business Operations SAMSON PHARMACEUTICAL 088169581 MANUFACTURE(20146-7000)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.