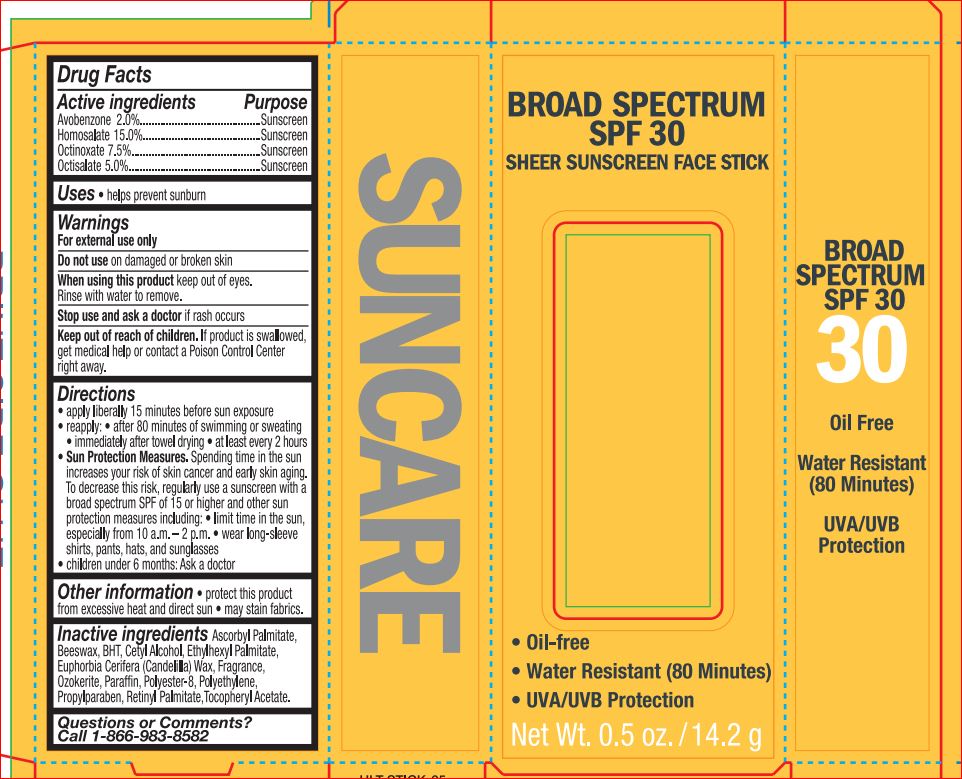
SPF 30 SHEER SUNSCREEN FACE ULTA- avobenzone 2.00% homosalate 15.00% octinoxate 7.50% octisalate 5.00% stick
SPF 30 Sheer sunscreen face by
Drug Labeling and Warnings
SPF 30 Sheer sunscreen face by is a Otc medication manufactured, distributed, or labeled by Ulta, Product Quest Mfg. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active ingredients Purpose
Avobenzone 2.00%........................................................................Sunscreen
Octisalate 3.00%............................................................................Sunscreen
Octocrylene 7.00%.........................................................................Sunscreen
Oxybenzone 3.00%........................................................................Sunscreen - PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Flammable: Do not use near heat, flame, or while smoking.
Do not use on damaged or broken skin
When using this product Keep out of eyes. Rinse eyes with water to remove.
Keep away from face to avoid breathing it Do not puncture or incinerate.
Contents under pressure. Do not store at temperatures above 120ºF.
Stop use and ask a doctor if rash occurs - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
apply liberally 15 minutes before sun exposure
reapply:
after 80 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk
of skin cancer and early skin aging. To decrease this risk, regularly use
a sunscreen with a broad spectrum SPF of 15 or higher and other
sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
children under 6 months: Ask a doctor - Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPF 30 SHEER SUNSCREEN FACE ULTA
avobenzone 2.00% homosalate 15.00% octinoxate 7.50% octisalate 5.00% stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62296-7778 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) Avobenzone 2 g in 100 g Homosalate (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) Homosalate 15 g in 100 g Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 7.5 g in 100 g Octisalate (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) Octisalate 5 g in 100 g Inactive Ingredients Ingredient Name Strength Ascorbyl Palmitate (UNII: QN83US2B0N) YELLOW WAX (UNII: 2ZA36H0S2V) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Cetyl Alcohol (UNII: 936JST6JCN) Ethylhexyl Palmitate (UNII: 2865993309) CANDELILLA WAX (UNII: WL0328HX19) CERESIN (UNII: Q1LS2UJO3A) PARAFFIN (UNII: I9O0E3H2ZE) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Propylparaben (UNII: Z8IX2SC1OH) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62296-7778-1 4.2 g in 1 TUBE; Type 0: Not a Combination Product 01/27/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/27/2016 Labeler - Ulta (608168597) Registrant - Product Quest Mfg (927768135) Establishment Name Address ID/FEI Business Operations Product Quest Mfg 927768135 manufacture(62296-7778) , label(62296-7778)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.