ZORBTIVE- somatropin kit
Zorbtive by
Drug Labeling and Warnings
Zorbtive by is a Prescription medication manufactured, distributed, or labeled by EMD Serono, Inc., Merck Serono, S.A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZORBTIVE® safely and effectively. See full prescribing information for ZORBTIVE®.
ZORBTIVE® (somatropin) for injection, for subcutaneous use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
Zorbtive® is a recombinant human growth hormone indicated for the treatment of short bowel syndrome in adult patients receiving specialized nutritional support. (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage is 0.1 mg/kg subcutaneously once daily to a maximum daily dose of 8 mg for 4 weeks. (2.1)
- See full prescribing information for information on dosage titration and management of fluid retention and arthralgia/carpal tunnel syndrome (2.1); and step-by-step instructions on preparation and administration of the injection. (2.2, 2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 8.8 mg, lyophilized powder in a single-patient use vial for reconstitution (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Neoplasms: Monitor patients for potential malignant changes of pre-existing nevi; discontinue if there is evidence of recurrent activity. (4, 5.1)
- Acute Critical Illness: Increased mortality due to complication following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure. (4, 5.2)
- Impaired Glucose Tolerance/Diabetes Mellitus (new onset or exacerbation): Monitor blood glucose and adjust concurrent antidiabetic treatment, as needed. (5.3)
- Hypersensitivity Reactions: Serious hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention. (4, 5.4)
- Lipoatrophy: Rotate injection sites. (2.3, 5.5)
- Fluid Retention: Tissue turgor and musculoskeletal discomfort may resolve spontaneously or require analgesic treatment or a reduction in Zorbtive® dose. (2.1, 5.6)
- Carpal Tunnel Syndrome: Decrease the dosage of Zorbtive®, discontinue treatment if symptoms do not resolve. (2.1, 5.7)
- Pancreatitis: Consider pancreatitis in any patient who develops abdominal pain. (5.8)
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism. (5.9, 7.1)
- Hypothyroidism: Evaluate thyroid function in patients with suspected and/or diagnosed hypopituitarism before starting Zorbtive® and again following 4 weeks of treatment; correct thyroid function, if needed. (5.10)
- Intracranial Hypertension: Exclude preexisting papilledema. (5.11)
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative: Zorbtive® is not approved for use in pediatric patients (5.12, 8.4)
ADVERSE REACTIONS
Most common adverse reactions (> 20%) are: peripheral edema, facial edema, arthralgia, injection site pain, flatulence, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at 1-800-283-8088 ext. 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Cytochrome P450-Metabolized Drugs (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine): Monitor clinical response to the CYP-metabolized drug, as dose adjustments may be required. (7.2)
- Oral Estrogen: Monitor patients for lack of efficacy of Zorbtive®. (7.3)
- Insulin and/or Oral/Injectable Hypoglycemic Agents: Adjust antidiabetic treatment, as needed. (7.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation Instructions
2.3 Administration Instructions
2.4 Storage of Diluted Solution
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neoplasms
5.2 Acute Critical Illness
5.3 Impaired Glucose Tolerance/Diabetes Mellitus
5.4 Hypersensitivity Reactions
5.5 Lipoatrophy
5.6 Fluid Retention and Arthralgia
5.7 Carpal Tunnel Syndrome
5.8 Pancreatitis
5.9 Hypoadrenalism
5.10 Hypothyroidism
5.11 Intracranial Hypertension
5.12 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Glucocorticoids
7.2 Cytochrome P450-Metabolized Drugs
7.3 Oral Estrogen
7.4 Insulin and/or Other Oral/Injectable Hypoglycemic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of Zorbtive® in adults is 0.1 mg/kg subcutaneously once daily to a maximum daily dose of 8 mg for 4 weeks.
Dosage Titration for Fluid Retention and Arthralgia/Carpal Tunnel Syndrome [see Warnings and Precautions (5.6, 5.7)]
- For moderate toxicity, treat symptomatically with analgesics or reduce the dosage of Zorbtive® to 0.05 mg/kg (maximum total dose of 4 mg) subcutaneously once daily.
- For severe toxicity, discontinue Zorbtive® for up to 5 days. Upon resolution of symptoms, resume Zorbtive® at 0.05 mg/kg (maximum total dose of 4 mg) subcutaneously once daily. Permanently discontinue Zorbtive®, if severe toxicity recurs or does not disappear within 5 days.
2.2 Preparation Instructions
Zorbtive® is provided in a package that contains all items required to reconstitute and inject the drug [see How Supplied/Storage and Handling (16)]. Prepare Zorbtive® using the following steps.
- 1. Determine the volume needed for the prescribed dosage based on the patient's weight and recommended dosage. Patient weight should be rounded to the nearest kilogram when determining dose. Table 1 below provides the volume of diluent to be added for the reconstitution and concentration of the reconstituted solution for Zorbtive® vial 8.8 mg.
Table 1: Reconstitution of Injection Adult Patient Weight (kg) Volume of diluent to be added for the reconstitution (mL) Concentration of reconstituted solution (mg/mL) More than 44 kg 1 mL 8.8 mg/mL Less than or equal to 44 kg 2 mL 4.4 mg/mL - 2. Use appropriate aseptic technique when preparing and administering Zorbtive®.
- 3. Reconstitute the vial(s) of Zorbtive® with either 1 mL or 2 mL of Bacteriostatic Water for Injection, USP (containing Benzyl Alcohol) using a 3 mL syringe. Inject the appropriate amount of diluent into the vial of Zorbtive® aiming the liquid against the glass vial wall. For patients unable to receive Benzyl Alcohol, Zorbtive® may be reconstituted with Sterile Water for Injection, USP [see Warnings and Precautions (5.12)].
- 4. Gently swirl the vial in circles until the contents are dissolved completely. DO NOT SHAKE.
- 5. Visually inspect the reconstituted solution. The final reconstituted solution should be clear and colorless. Discard the vial if the solution is cloudy or contains particulate matter.
2.3 Administration Instructions
- Withdraw the required volume of the reconstituted solution using a 1 mL syringe with a 29-gauge needle.
- Inject the full contents of the syringe subcutaneously at a 90° angle into the top side of the thigh, the areas around the belly button, the back of the upper arms, and the buttocks or hips.
- Rotate injection sites and do not give injections into areas where the skin is tender, bruised, red, or hard.
2.4 Storage of Diluted Solution
Once reconstituted with Bacteriostatic Water for Injection, USP (containing Benzyl Alcohol), Zorbtive® should be stored under refrigeration (2 to 8°C/36 to 46°F) and for no more than 14 days. Do not freeze. If Zorbtive® is reconstituted with Sterile Water for Injection, USP, the reconstituted solution should be used immediately and any unused solution should be discarded.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Zorbtive® is contraindicated in patients with:
- active malignancy
- acute critical illness due to complications following open heart or abdominal surgery, multiple accidental trauma or acute respiratory failure [see Warnings and Precautions (5.2)]
- active proliferative or severe non-proliferative diabetic retinopathy
- hypersensitivity to somatropin or any of the excipients of Zorbtive®. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Neoplasms
Zorbtive® is contraindicated in patients with active malignancy. Any preexisting malignancy should be inactive and its treatment complete prior to instituting therapy with somatropin. Discontinue somatropin if there is evidence of recurrent activity [see Contraindications (4)].
In childhood cancer survivors, an increased risk of a second neoplasm has been reported in patients treated with somatropins after their first neoplasm. Intracranial tumors, in particular meningiomas, in patients treated with radiation to the head for their first neoplasm, were the most common of these second neoplasms. In adult cancer survivors, the risk of occurrence is unknown. Given the limited data available, patients under growth hormone therapy should be carefully monitored for progression or recurrence of the tumor.
The safety and effectiveness of Zorbtive® in the treatment for short bowel syndrome in pediatric patients have not been established and Zorbtive® is not approved for use in pediatric patients.
Monitor patients on somatropin therapy carefully for potential malignant changes of preexisting nevi.
5.2 Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic amounts of somatropin [see Contraindications (4)]. Two placebo-controlled clinical trials in non-growth hormone deficient adult patients (n=522) with these conditions in intensive care units revealed a significant increase in mortality (42% vs. 19%) among somatropin-treated patients (doses 5.3 to 8 mg per day) compared to those receiving placebo. Discontinue Zorbtive® if the patient has acute critical illness.
5.3 Impaired Glucose Tolerance/Diabetes Mellitus
The use of somatropins have been associated with cases of new onset impaired glucose intolerance, new onset type 2 diabetes mellitus and exacerbation of preexisting diabetes mellitus. Some patients developed diabetic ketoacidosis and diabetic coma. In some patients, these conditions improved when the drug was discontinued, while in others the glucose intolerance persisted. Some patients necessitated initiation or adjustment of antidiabetic treatment (e.g., insulin and/or other oral/injectable hypoglycemic agents) while on somatropin. Monitor blood glucose in patients with other risk factors for glucose intolerance during Zorbtive® therapy and adjust antidiabetic treatment, as needed.
5.4 Hypersensitivity Reactions
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products. Inform patients and caregivers that such reactions are possible and to seek prompt medical attention if a hypersensitivity reaction occurs. [see Contraindications (4)].
5.5 Lipoatrophy
When somatropins are administered subcutaneously at the same site over a long period of time, tissue atrophy may result. Avoid tissue atrophy by rotating the injection site [see Dosage and Administration (2.3)].
5.6 Fluid Retention and Arthralgia
Increased fluid retention resulting in tissue turgor (swelling, particularly in the hands and feet) and arthralgia resulting in musculoskeletal discomfort (pain, swelling and/or stiffness) may occur during treatment with Zorbtive®, but may resolve spontaneously or with analgesic therapy or after reducing the dosage [see Dosage and Administration (2.1)].
5.7 Carpal Tunnel Syndrome
Carpal tunnel syndrome may occur during treatment with somatropin. If the symptoms of carpal tunnel syndrome do not resolve by decreasing the dosage of Zorbtive®, discontinue treatment [see Dosage and Administration (2.1)].
5.8 Pancreatitis
Cases of pancreatitis have been reported in pediatric patients and adults receiving somatropin treatment, with some evidence supporting a greater risk in pediatric patients compared with adults. Published literature indicates that females who have Turner syndrome may be at greater risk than other somatropin-treated pediatric patients. Pancreatitis should be considered in any somatropin-treated patient, especially a pediatric patient who develops abdominal pain. The safety and effectiveness of Zorbtive® in the treatment for short bowel syndrome in pediatric patients have not been established and Zorbtive® is not approved for use in pediatric patients.
5.9 Hypoadrenalism
Patients receiving somatropin therapy who have or are at risk for pituitary homone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Zorbtive® therapy [see Drug Interactions (7.1)].
5.10 Hypothyroidism
Growth hormone can affect the metabolism of thyroid hormones by increasing the extrathyroidal conversion of T4 to T3 and this lowering effect on T4 may unmask incipient central hypothyroidism in hypopituitary patients. Evaluate thyroid function in patients with suspected and/or diagnosed hypopituitarism before starting Zorbtive® therapy and again following 4 weeks of treatment. If hypothyroidism is diagnosed following a course of Zorbtive® therapy, it should be corrected.
5.11 Intracranial Hypertension
No cases of intracranial hypertension (IH) have been observed among patients with short bowel syndrome treated with Zorbtive®. The syndrome of intracranial hypertension (IH), with papilledema, visual changes, headache, and nausea and/or vomiting has been reported in a small number of pediatric patients with growth failure treated with somatropins. Symptoms usually occurred within the first 8 weeks after the initiation of somatropin therapy. IH-associated signs and symptoms usually resolved after cessation of therapy or a reduction of the somatropin dosage. The safety and effectiveness of Zorbtive® in the treatment for short bowel syndrome in pediatric patients have not been established and Zorbtive® is not approved for use in pediatric patients.
Funduscopic evaluation of patients is recommended at the initiation of Zorbtive® therapy and if patients present with symptoms of IH. If papilledema is observed by funduscopy during Zorbtive® treatment, discontinue treatment.
5.12 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
Zorbtive® is not approved for use in neonates or infants. Serious and fatal adverse reactions including "gasping syndrome" can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs, including Zorbtive® when reconstituted with Bacteriostatic Water for Injection, USP. The "gasping syndrome" is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (when reconstituted with Bacteriostatic Water for Injection, USP, Zorbtive® contains 9 mg of benzyl alcohol per mL) [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Neoplasms [see Warnings and Precautions (5.1)]
- Acute Critical Illness [see Warnings and Precautions (5.2)]
- Impaired Glucose Intolerance/Diabetes Mellitus [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Lipoatrophy [see Warnings and Precautions (5.5)]
- Fluid Retention and Arthralgia [see Warnings and Precautions (5.6)]
- Carpel Tunnel Syndrome [see Warnings and Precautions (5.7)]
- Pancreatitis [see Warnings and Precautions (5.8)]
- Hypoadrenalism [see Warnings and Precautions (5.9)]
- Hypothyroidism [see Warnings and Precautions (5.10)]
- Intracranial Hypertension [see Warnings and Precautions (5.11)]
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot always be directly compared to the rates in the clinical trials of another drug, and may not reflect the adverse reaction rates observed in practice.
In a double-blind, randomized, placebo-controlled clinical trial, 32 patients were exposed to Zorbtive® for 4 weeks. Of the 41 patients enrolled in the trial, 16 patients received Zorbtive® (0.1 mg/kg per day) plus supportive oral diet, 16 patients received subcutaneous Zorbtive® (0.1 mg/kg per day) plus supportive oral diet plus oral glutamine (30 grams per day), and 9 patients received placebo with specialized oral diet and oral glutamine (30 grams per day).
The most common adverse reactions occurring in greater than 20% of patients treated with Zorbtive® alone and at a higher frequency than in the control group include peripheral edema, facial edema, arthralgia, injection site pain, flatulence, and abdominal pain.
Table 2 summarizes adverse reactions that occurred in at least 10% of patients receiving Zorbtive®, alone or in combination with glutamine and at a higher incidence than in the control group.
Table 2: Adverse Reactions* in a Randomized, Placebo Controlled Trial of Zorbtive® in Adult Patients with Short Bowel Syndrome: 4 Week Treatment Period Treatment Group* Adverse Reaction Zorbtive® alone Zorbtive® + Glutamine Control (Placebo + Glutamine) n=16
n (%)n=16
n (%)n=9
n (%)- * occurring at in ≥10% of Zorbtive® -treated patients and at a higher incidence than control
Peripheral edema 11 (69) 13 (81) 1 (11) Facial edema 8 (50) 7 (44) 0 (0) Arthralgia 7 (44) 5 (31) 0 (0) Injection site pain 5 (31) 0 (0) 0 (0) Flatulence 4 (25) 4 (25) 2 (22) Abdominal pain 4 (25) 2 (13) 1 (11) Injection site reaction 3 (19) 4 (25) 1 (11) Vomiting 3 (19) 3 (19) 1 (11) Pain 3 (19) 1 (6) 1 (11) Nausea 2 (13) 5 (31) 0 (0) After 4 weeks of treatment with Zorbtive® patients were discharged for follow-up on a supportive oral diet supplemented either with glutamine or glutamine placebo, and subjects were re-evaluated as outpatients 12 weeks later. No new adverse drug reactions were observed in the follow up period.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Zorbtive® or other somatropin products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Zorbtive®:
- headache
- gynecomastia
Other somatropin products:
- new onset impaired glucose tolerance, new onset type 2 diabetes mellitus, exacerbation of preexisting diabetes mellitus, diabetic ketoacidosis, diabetic coma [see Warnings and Precautions (5.3)]
- serious systemic hypersensitivity reactions, including anaphylaxis and angioedema [see Warnings and Precautions (5.4)].
- pancreatitis [see Warnings and Precautions 5.8)]
- leukemia
-
7 DRUG INTERACTIONS
7.1 Glucocorticoids
The microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. Somatropin inhibits 11βHSD-1 which can lead to reduced serum cortisol concentrations. As a consequence, in patients treated with Zorbtive®, previously undiagnosed secondary (central) hypoadrenalism may be unmasked and glucocorticoid replacement may be required in patients treated with Zorbtive®. In addition, patients treated with glucocorticoid replacement therapy for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following the initiation of Zorbtive® therapy [see Warnings and Precautions (5.9)]. However, formal drug interaction studies have not been conducted.
7.2 Cytochrome P450-Metabolized Drugs
Limited published data indicate that somatropin treatment increases cytochrome P450 (CYP450)-mediated antipyrine clearance in humans, which involves multiple isozymes including CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C18, and CYP3A4. These data suggest that somatropin administration may alter the clearance of compounds metabolized by CYP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine). When somatropin is administered in combination with these drugs, monitor clinical response to the CYP-metabolized drug, as dosage adjustments may be required. However, formal drug interaction studies have not been conducted.
7.3 Oral Estrogen
Because oral estrogens may reduce the serum IGF-1 response to somatropin treatment, females receiving oral estrogen replacement may have reduced efficacy from Zorbtive®. However, formal drug interaction studies have not been conducted. Monitor patients taking oral estrogens for lack of efficacy of Zorbtive®.
7.4 Insulin and/or Other Oral/Injectable Hypoglycemic Agents
Patients with diabetes mellitus who receive concomitant antidiabetic treatment with Zorbtive® may require adjustment of their doses of insulin and/or other hypoglycemic agents [see Warnings and Precautions (5.3)]. However, formal drug interaction studies have not been conducted.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Zorbtive® use in pregnant women to inform any drug associated risks. In animal reproduction studies, no fetal harm was reported with subcutaneous administration of somatropin during the period of organogenesis in rats and rabbits at doses of approximately up to 5 and 10 times, respectively, the recommended human dose of 0.1 mg/kg/day [see Data]. The estimated background risk of major birth defects and miscarriages for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Reproduction studies of somatropin have been performed in rats and rabbits. Administration of somatropin to rats and rabbits during the period of organogenesis at subcutaneous doses of approximately up to 5 and 10 times the recommended human dosage of 0.1 mg/kg/day, based on body surface area, respectively, have revealed no evidence of harm to the fetus due to somatropin. In a pre- and post-natal development study in rats, subcutaneous doses of approximately up to 5 times the recommended human dosage of 0.1 mg/kg/day (based on body surface area) had no adverse effect on pre- and post-natal development.
8.2 Lactation
Risk Summary
There are no data on the presence of somatropin in human milk. Limited published literature reports no adverse effects on breastfed infants with maternal administration of somatropin. No decrease in milk production or change in milk content during treatment with somatropin has been reported. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Zorbtive® and any potential adverse effects on the breastfed infant from Zorbtive® or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Zorbtive® in the treatment for short bowel syndrome in pediatric patients have not been established.
Zorbtive® is contraindicated in patients with active malignancy. In pediatric cancer survivors, an increased risk of a second neoplasm has been reported in patients treated with somatropins after their first neoplasm. Intracranial tumors, in particular meningiomas, in patients treated with radiation to the head for their first neoplasm, were the most common of these second neoplasms [see Warnings and Precautions (5.1)].
Cases of pancreatitis have been reported in patients receiving somatropin treatment, with some evidence supporting a greater risk in pediatric patients compared with adults, particularly females with Turner syndrome [see Warnings and Precautions (5.8)].
The syndrome of intracranial hypertension (IH), with papilledema, visual changes, headache, and nausea and/or vomiting has been reported in a small number of pediatric patients with growth failure treated with somatropins [see Warnings and Precautions (5.11)].
Zorbtive® is not approved for use in neonates or infants. Serious adverse reactions including fatal reactions and the "gasping syndrome" occurred in premature neonates and low birth weight infants in the neonatal intensive care unit who received benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (when reconstituted with Bacteriostatic Water for Injection, USP, Zorbtive® contains 9 mg of benzyl alcohol per mL) [see Warnings and Precautions (5.12)].
Zorbtive® is a recombinant human growth hormone and therefore may increase growth and cause growth-related problems (e.g. slipped capital femoral epiphysis) in the patients receiving it, particularly pediatric patients whose epiphyses are not yet closed.
8.5 Geriatric Use
Clinical studies with Zorbtive® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly patients may be more sensitive to growth hormone action, and may be more prone to develop adverse reactions. Thus, dose selection for an elderly patient should be cautious, usually starting at a lower dose.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Zorbtive® (somatropin) for injection is a human growth hormone produced by recombinant DNA technology for subcutaneous use. Zorbtive® has 191 amino acid residues and a molecular weight of 22,125 daltons. Its amino acid sequence and structure are identical to the dominant form of human pituitary growth hormone. Zorbtive® is produced by a mammalian cell line (mouse C127) that has been modified by the addition of the human growth hormone gene. Zorbtive® is secreted directly through the cell membrane into the cell-culture medium for collection and purification.
Zorbtive® is a highly purified preparation. Biological potency is determined by measuring the increase in the body weight induced in hypophysectomized rats.
Zorbtive® is a sterile, lyophilized powder available in 8.8 mg vials for single-patient use. Each 8.8 mg vial contains 8.8 mg somatropin, 2.05 mg phosphoric acid and 60.19 mg sucrose.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Intestinal mucosa contains receptors for growth hormone and for insulin-like growth factor-1 (IGF-1), which is known to mediate many of the cellular actions of growth hormone. Thus, the actions of somatropin on the gut may be direct or mediated via the local or systemic production of IGF-1.
In human clinical studies the administration of growth hormone has been shown to enhance the transmucosal transport of water, electrolytes, and nutrients.
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of somatropin after subcutaneous administration was 70% to 90%. The mean half-life (t½) after subcutaneous administration is approximately 4 hours compared to 0.6 hours following intravenous administration in male healthy subjects down-regulated with somatostatin, indicating that the subcutaneous absorption of somatropin is a rate-limiting process. No significant accumulation of somatropin appears to occur after 4 weeks of subcutaneous daily dosing as indicated.
Distribution
The steady-state volume of distribution (Mean ± SD) following intravenous administration of Zorbtive® in healthy subjects was 12 ± 1 L.
Elimination
Somatropin clearance involves both linear and nonlinear, i.e., concentration-dependent, components. The t½ (Mean ± SD) in nine patients with HIV-associated wasting with an average weight of 57 ± 7 kg, given a 6 mg dose of somatropin subcutaneously was 4 ± 2 hrs.
Specific Populations
Age: Pediatric Population
Available evidence suggests that clearance of somatropin is similar in adults and pediatric patients, but no pharmacokinetic studies have been conducted in pediatric patients with short bowel syndrome.
Sex
Literature indicates that a sex-related difference in the mean clearance of somatropins exists (clearance of somatropin is greater in males compared to females). However, no sex-based analysis is available in healthy subjects or patients with short bowel syndrome.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to assess the carcinogenic potential of Zorbtive®. Somatropin was not genotoxic in in vitro and in vivo genotoxicity studies.
Subcutaneous administration of somatropin to male and female rats at doses up to approximately 5 times the human dosage of 0.1 mg/kg/day (based on body surface area) revealed no evidence of impairment of fertility or early embryonic development.
-
14 CLINICAL STUDIES
The efficacy of Zorbtive® was evaluated in a double-blind, randomized, placebo-controlled clinical trial in 41 adult patients with short bowel syndrome (SBS). Patients were 18 to 75 years of age, 12 (29%) were male, and 32 (78%) were White. The patients were randomized into three groups. The first treatment group (n=16) received Zorbtive® (0.1 mg/kg per day) plus specialized oral diet (high carbohydrate, low-fat diet, adjusted for individual patient requirements and preferences), and the second treatment group (n=16) received Zorbtive® (0.1 mg/kg per day) plus specialized oral diet plus oral glutamine (30 grams per day). The control group (n= 9) received placebo with specialized oral diet and oral glutamine (30 grams per day). Study treatment was administered subcutaneously once daily for 4 weeks. After completion of 4 weeks of treatment, patients were discharged for follow-up on a supportive oral diet supplemented either with glutamine or glutamine placebo, and patients were re-evaluated as outpatients 12 weeks later.
The primary endpoint of the study was the change in weekly total intravenous parenteral nutrition (IPN) volume defined as the sum of the volumes of IPN, supplemental lipid emulsion (SLE), and intravenous hydration fluid. The secondary endpoints were the change in weekly IPN caloric content and the change in the frequency of IPN administration per week. The mean baseline IPN volume, mean IPN caloric content, and mean frequency of IPN administration are provided in Table 3. Mean reductions in IPN volume, IPN caloric content and the frequency of IPN administration in each patient group were significantly greater in both Zorbtive®-treated groups than in the control group.
Table 3: Randomized, Placebo Controlled Trial of Zorbtive® in Adult Patients with Short Bowel Syndrome: Results for Endpoints after 4 Weeks of Treatment Zorbtive® alone Zorbtive® + Glutamine Control (Placebo + Glutamine) Total IPN volume (L/wk) Mean at Baseline 10.3 10.5 13.5 Mean Change -5.9 -7.7 -3.8 Treatment Differences (with control) -2.1* -3.9** Total IPN Calories (kcal/wk) Mean at Baseline 7634.7 7895.0 8570.4 Mean Change -4338.3 -5751.2 -2633.3 Treatment Differences (with control) -1705.0 -3117.9 Frequency of IPN or SLE (days/wk) Mean at Baseline 5.1 5.4 5.9 Mean Change -3.0 -4.2 -2.0 Treatment Differences (with control) -1.0 -2.2 -
16 HOW SUPPLIED/STORAGE AND HANDLING
Zorbtive® is packaged as NDC: 44087-3388-7:
- single-patient use vials of Zorbtive®, each containing 8.8 mg of somatropin as a white lyophilized powder for injection
- single-patient use vials of Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Glucose Intolerance/Diabetes Mellitus
Inform patients that new onset impaired glucose intolerance/diabetes mellitus or exacerbation of preexisting diabetes mellitus can occur and monitoring of blood glucose during treatment with Zorbtive® may be needed [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform patients that local or systemic reactions are possible. Instruct patients to contact their healthcare provider should they experience any side effects or discomfort during treatment with Zorbtive® [see Warnings and Precautions (5.4)].
Administration
Administer Zorbtive® using sterile, disposable syringes and needles. Instruct patient in the importance of proper disposal and cautioned against any reuse of needles and syringes. An appropriate container for the disposal of used syringes and needles should be employed.
Instruct patients to rotate injection sites to avoid localized tissue atrophy [see Warnings and Precautions (5.5)].
Refer patients to the Instructions for Use on how to prepare and administer an injection of Zorbtive.
Fluid Retention/Carpel Tunnel Syndrome
Inform patients that increased tissue turgor (swelling, particularly in the hands and feet) and musculoskeletal discomfort (pain, swelling and/or stiffness) may occur during treatment with Zorbtive® and to report to their healthcare provider any signs or symptoms that occur during treatment with Zorbtive® [see Warnings and Precautions (5.6 and 5.7)].
Pancreatitis
Inform patients that pancreatitis may develop and to report to their healthcare provider any new onset abdominal pain [see Warnings and Precautions (5.8)].
Hypoadrenalism
Inform patients who have or who are at risk for pituitary hormone deficiency(s) that hypoadrenalism may develop and to report to their healthcare provider if they experience hyperpigmentation, extreme fatigue, dizziness, weakness, or weight loss [see Warnings and Precautions (5.9)].
Hypothyroidism
Inform patients that hypothyroidism may develop and that their thyroid function may be monitored before starting Zorbtive® and again following 4 weeks of treatment [see Warnings and Precautions (5.10)].
Intracranial Hypertension
Instruct patients to report to their healthcare provider any visual changes, headache, and nausea and/or vomiting [see Warnings and Precautions (5.11)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Manufactured for: EMD Serono, Inc., Rockland, MA 02370 For more information call the AXIS® Center at 1-877-714-2847. This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: September 2019Patient Information
Zorbtive® (Zorb-tiv)
(somatropin) for injection
for subcutaneous useWhat is Zorbtive®?
Zorbtive® is a prescription medicine used to treat adults with short bowel syndrome who are receiving a special diet.
It is not known if Zorbtive® is safe and effective for use as a treatment for children with short bowel syndrome.Who should not use Zorbtive®?
Do not use Zorbtive® if you:- have cancer
- have a serious medical condition after having:
- open heart surgery
- stomach area (abdominal) surgery
- an accident (trauma)
- severe breathing problems (respiratory failure)
- have eye problems caused by diabetes (active proliferation or severe non-proliferative diabetic retinopathy)
- are allergic to any of the ingredients in Zorbtive®. See the end of this leaflet for a complete list of ingredients in Zorbtive®.
Before using Zorbtive®, tell your healthcare provider about all of your medical conditions, including if you: - had cancer
- have diabetes or blood sugar problems
- have or had numbness and tingling in your wrist and hand (carpal tunnel syndrome)
- have thyroid problems (hypothyroidism)
- have pancreas problems
- have kidney or liver problems
- are pregnant or plan to become pregnant. It is not known if Zorbtive® will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Zorbtive® passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using Zorbtive®.
Zorbtive® and other medicines may affect each other causing serious side effects. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider when you get a new medicine.How should I use Zorbtive®? - See the Instructions for Use at the end of this leaflet for detailed instructions about how to inject Zorbtive® the right way. Do not use Zorbtive® until your healthcare provider has shown you how to inject Zorbtive® the right way.
- Use Zorbtive® exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much Zorbtive® to use and when to use it. Your healthcare provider may change your dose if needed.
- Zorbtive® is injected under the skin (subcutaneously) 1 time a day for 4 weeks. Your healthcare provider will tell you where to inject Zorbtive®. Some examples of common injection sites include the top side of the thigh, the areas around the belly button, the back of the upper arms, and the buttocks or hips.
- Change (rotate) your injection site with each injection. Do not inject Zorbtive® into skin that is tender, bruised, red, or hard.
- Always use a new, unopened, needle and syringe for each injection. Never reuse needles and syringes.
- Throw away used needles and syringes in a FDA approved sharps container. See "Disposing of used needles and syringes" in the Zorbtive® Instructions for Use.
What are the possible side effects of Zorbtive®?
Zorbtive® may cause serious side effects, including:- return of tumor or cancerous growth. If you had cancer in the past, using Zorbtive® may cause your cancer to come back. See "Who should not use Zorbtive®" above.
- diabetes or blood sugar problems. Zorbtive® can cause diabetes or blood sugar problems that do not go away after you stop using Zorbtive®. Zorbtive® can also cause your diabetes and blood sugar problems that you already have to get worse. Your healthcare provider may need to check your blood sugar or change your diabetes medicines while you are using Zorbtive®.
- serious allergic reactions. Stop using Zorbtive® and go to the nearest hospital emergency room right away if you have any signs or symptoms indicating anaphylactic shock which may include fainting, confusion, trouble swallowing, and difficulty breathing.
- injection site reactions (atrophy). Zorbtive® may cause redness, itching, and tissue weakness in the area of skin you injected. Changing (rotating) your injection site each time may lower the chances of injection site atrophy.
- swelling of your hands and feet (fluid retention). Call your healthcare provider if you have any swelling of your hands and feet.
- numbness and tingling in your wrist and hand (carpal tunnel syndrome).
- decrease in thyroid hormone levels in your blood (hypothyroidism). Your healthcare provider will do blood tests to check your thyroid function before you start and 4 weeks after you stop using Zorbtive®.
- decreased function of adrenal glands (hypoadrenalism). Call your healthcare provider if you experience changes in skin color, extreme tiredness, dizziness, weakness, or unexplained weight loss.
- pancreatitis. Call your healthcare provider or go to the nearest hospital emergency room right away if you have any signs or symptoms of sudden pancreatitis including:
- severe pain in the upper stomach area
- sweating, nausea, vomiting
- skin and whites of the eyes turn yellow
- swollen and tender stomach area
- fever
- fast heart beat
- increased pressure in your skull (intracranial hypertension). Your healthcare provider may test your eyes while you use Zorbtive®. Call your healthcare provider or go to the nearest hospital emergency room right away if you have any signs or symptoms of intracranial hypertension including:
- headaches
- nausea or vomiting
- vision changes
- benzyl alcohol toxicity. Benzyl alcohol is a preservative in the Bacteriostatic Water for Injection (water for mixing the multi-dose vial of Zorbtive®). Benzyl alcohol has caused serious side effects, including death, in children, especially premature and low-birth weight infants who received the preservative benzyl alcohol. Ask your healthcare provider or pharmacist for plain sterile water for mixing with Zorbtive® if you are allergic to benzyl alcohol.
The most common side effects of Zorbtive® include: - swelling of the arms, legs, hands, feet, face
- gas
- nausea
- stomach area (abdominal), injection site pain
- muscle pain and stiffness
- injection site reactions
- vomiting
- pain
These are not all the possible side effects of Zorbtive®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store Zorbtive®? - Store unmixed vials of Zorbtive®, Sterile Water, and Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol), at room temperature between 59°F to 86°F (15°C to 30°C).
- Store Zorbtive® mixed with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 14 days (2 weeks).
- Zorbtive® mixed with Sterile Water should be used right away. Throw away any unused Zorbtive® mixed with Sterile Water. Do not store it for later use.
- Do not freeze mixed Zorbtive®.
- Do not use Zorbtive® past the expiration date printed on the carton.
- Throw away medicine that is past the expiration date or no longer needed.
General information about the safe and effective use of Zorbtive®.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Zorbtive® for a condition for which it was not prescribed. Do not give Zorbtive® to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Zorbtive® that is written for health professionals.What are the ingredients in Zorbtive®?
Active ingredient: somatropin
Inactive ingredients: sucrose, phosphoric acid -
Instructions for Use
Zorbtive® (Zorb-tiv)
(somatropin) for injection
for subcutaneous useRead this Instructions for Use before you start using Zorbtive® and each time you get a new Zorbtive® vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your needles or syringes with another person. You may give other people a serious infection or other people may get a serious infection from you.
Supplies needed to give your Zorbtive® injection (See Figure A): Included in your Zorbtive® kit: 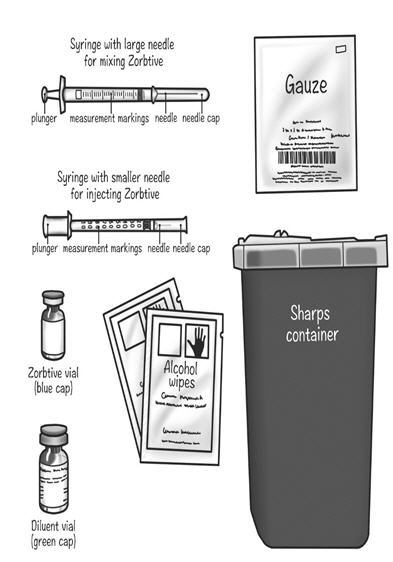
- 1 Zorbtive® vial
- 1 Bacteriostatic Water for Injection or Sterile Water (diluent)
- 1 3 mL syringe with large 1 inch needle attached (for mixing Zorbtive®)
- a 1 mL syringe with small 29-31 gauge needle attached (for giving your Zorbtive® injection)
- 2 alcohol wipes
- 1 gauze or cotton ball
- A clean flat surface like a table
- 1 sharps container for throwing away used needles and syringes. See "Disposing of used needles and syringes" at the end of these instructions.
Figure A Preparing your Zorbtive® dose:
- Prepare a clean flat surface, like a table or counter top
- Gather all the supplies you will need to give your Zorbtive® injection
- Wash your hands with soap and water
Step 1: Using your thumb, flip the plastic cap off of the Zorbtive® and diluent vial. 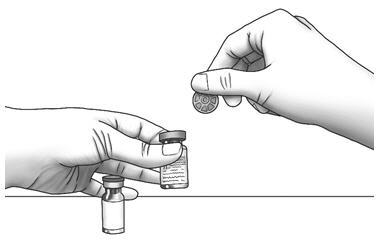
Step 2: Wipe the rubber stoppers with 1 alcohol wipe. 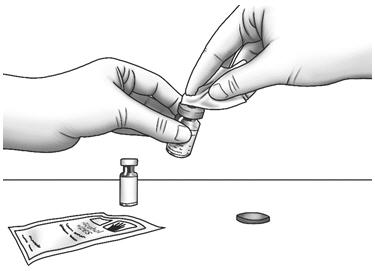
Step 3: Unwrap the 3 mL syringe with the large 1 inch needle attached. With the needle pointing upwards, pull the plunger back until the top of the plunger reaches the line slightly past the line for your prescribed dose. 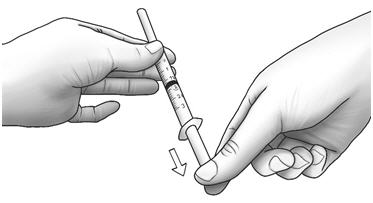
Step 4: Pull the needle cap straight off the syringe. 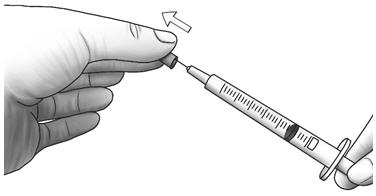
- Do not touch the needle or let the needle touch any surface.
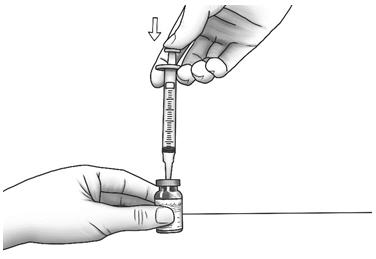
Step 5: With the needle pointing downward, hold the syringe straight over the diluent vial, then push the needle through the marked center circle on the rubber stopper of the diluent vial. - Slowly push the plunger all the way in to inject the air into the diluent vial.
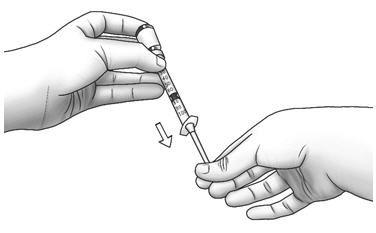
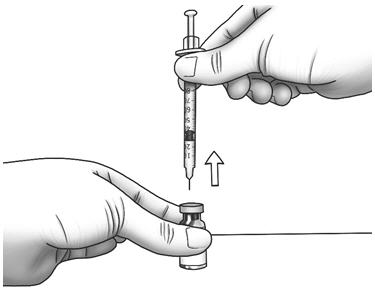
Step 6: Turn the vial upside down and slowly pull back on the plunger until the top of the plunger reaches the line slightly past the line for your prescribed dose. - If you see air bubbles, lightly tap the syringe with your fingertip.
- Slowly pull the needle out of the diluent vial.
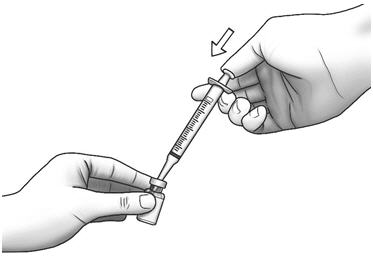
Step 7: With the needle pointing downward, hold the syringe straight over the Zorbtive® vial, then push the needle through the marked center on the rubber stopper on the Zorbtive® vial. - Point the needle tip toward the side of the Zorbtive® vial and then slowly push the plunger all the way in to inject the diluent into the Zorbtive® vial.
- Do not squirt the diluent directly onto the Zorbtive®. This can make your Zorbtive® foamy and hard to inject.
Step 8: Gently swirl the Zorbtive® vial in circles to mix Zorbtive® all the way. Do not shake the vial. - Pull the needle and syringe out of the vial and safely throw it away. See "Disposing of used needles and syringes" at the end of these instructions.
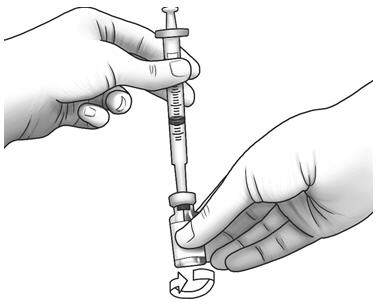
Step 9: Check the liquid in the Zorbtive® vial. The liquid should look clear and colorless. Do not use it if the liquid is discolored, cloudy, or contains any lumps or particles in it. Throw it away and tell your healthcare provider or pharmacist. - If the Zorbtive® liquid looks foamy, let the vial sit until the bubbles disappear.
Step 10: Unwrap the 3 mL syringe with the small 29-31 gauge needle attached. With the needle pointing upwards, pull back the plunger until the top of the plunger reaches the line slightly past the line for your prescribed dose. Step 11: Pull the cap straight off of the needle. - Do not touch the needle or let the needle touch any surface.
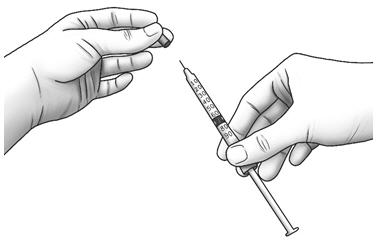
Step 12: Hold the Zorbtive® vial firmly on a flat surface, then push the needle through the marked center circle of the rubber stopper of the Zorbtive® vial. 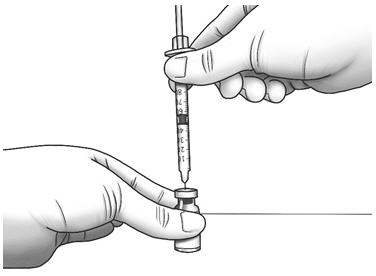
Step 13: Slowly push the plunger all the way in to inject air into the Zorbtive® vial. - Do not pull the needle out of the Zorbtive® vial.
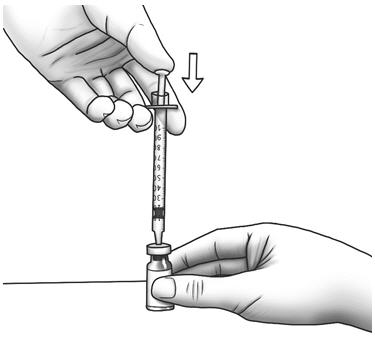
Step 14: Keeping the needle in the Zorbtive® vial, lift the vial and turn it upside down with the needle pointing toward the ceiling. With the needle tip still in the liquid, slowly pull the plunger back until the top of the plunger reaches the line for your prescribed dose. - Check the syringe to make sure that you have the right dose.
- If you see air bubbles, lightly tap the syringe with your fingertip.
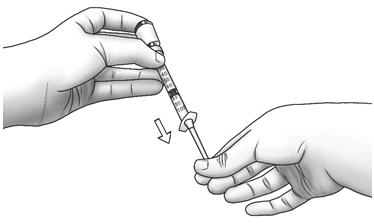
Step 15: Turn the Zorbtive® vial upright and pull the syringe straight out of the vial's rubber stopper. - Do not touch the needle or allow the needle to touch any surface.
Giving your Zorbtive® Injection:
- Inject Zorbtive® exactly under the skin into the areas recommended by your healthcare provider.
- Change (rotate) your injection site with each Zorbtive® injection.
- Do not inject Zorbtive® into skin that is tender bruised, red or hard.
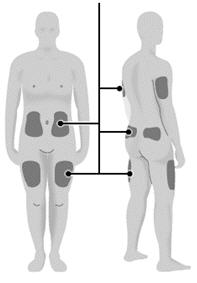
Step 16: Choose your injection site. - Select a new injection site. Examples of common injection sites include: the top side of the thigh, the areas around the belly button, the back of the upper arms, and the buttocks or hips.
- Choose a different injection site each time you inject to reduce redness, irritation, or other skin problems.
- Wipe the skin with an alcohol pad to clean the injection site and let it dry.
Step 17: Pinch the skin. Hold the syringe at a 90° angle and insert the needle into your skin. Step 18: Push the plunger all the way in to give your dose. 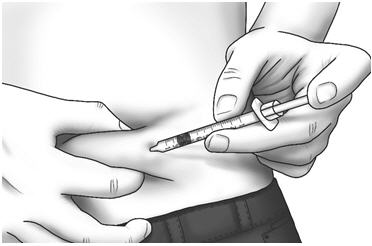
Step 19: Pull the needle straight out of your skin and safely throw it away. See "Disposing of used needles and syringes" at the end of these instructions. - If you see blood after you take the needle out of your skin, press your injection site with a piece of gauze or alcohol wipe.
- Do not recap the needle. Recapping the needle can lead to a needle stick injury.
Disposing of used needles and syringes:
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture- resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak- resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
General information about the safe and effective use of Zorbtive®
Keep Zorbtive® vials, syringes, needles, and all medicines out of the reach of children.
For further information or instruction assistance, you may call the AXIS® Center at 1-877-714-AXIS (2947).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: EMD Serono, Inc., Rockland, MA 02370
Revised: September 2019 - PRINCIPAL DISPLAY PANEL - 8.8 mg Vial Label
-
PRINCIPAL DISPLAY PANEL - 3.5 mL Vial Label
NDC: 44087-8007-0
Bacteriostatic Water for injection, USP
Sterile 3.5 mL Multiple Dose Vial
Rx Only
Each mL contains: Benzyl Alcohol (as bacteriostat) 0.9% in
Water for Injection q.s. For use as solvent vehicle or carrier of
drugs for parenteral use. Pyrogen free.NOT FOR USE
IN NEWBORNSStore at 15° to 30°C
(59° to 86°F)
[see USP Controlled
Room Temperature].Lot:
Exp:
L1320108D

-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 44087-3388-7
ZORBTIVE® 8.8 mg
(somatropin) for injection8.8 mg
For subcutaneous injection
Rx Only7 vials Zorbtive® (somatropin) for injection
7 vials Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol)EMD
SERONO
-
INGREDIENTS AND APPEARANCE
ZORBTIVE
somatropin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44087-3388 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44087-3388-7 1 in 1 CARTON 12/01/2003 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 7 VIAL, GLASS 7 mL Part 2 7 VIAL, GLASS 24.5 mL Part 1 of 2 ZORBTIVE
somatropin injection, powder, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 8.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 60.19 mg in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) 2.05 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021597 Part 2 of 2 BACTERIOSTATIC WATER
bacteriostatic water injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021597 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021597 12/01/2003 Labeler - EMD Serono, Inc. (088514898) Establishment Name Address ID/FEI Business Operations Merck Serono, S.A 480192579 MANUFACTURE(44087-3388)
Trademark Results [Zorbtive]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZORBTIVE 78976554 3064550 Live/Registered |
MERCK KGAA 2004-04-05 |
 ZORBTIVE 78976022 2974677 Dead/Cancelled |
Ares Trading, S.A. 2003-06-13 |
 ZORBTIVE 78396381 not registered Dead/Abandoned |
Ares Trading SA 2004-04-05 |
 ZORBTIVE 78262146 not registered Dead/Abandoned |
Ares Trading, S.A. 2003-06-13 |
 ZORBTIVE 77091013 not registered Dead/Abandoned |
Ares Trading S.A. 2007-01-25 |
 ZORBTIVE 76978149 3117798 Live/Registered |
MERCK KGAA 2004-09-09 |
 ZORBTIVE 76610553 not registered Dead/Abandoned |
Ares Trading S.A. 2004-09-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
