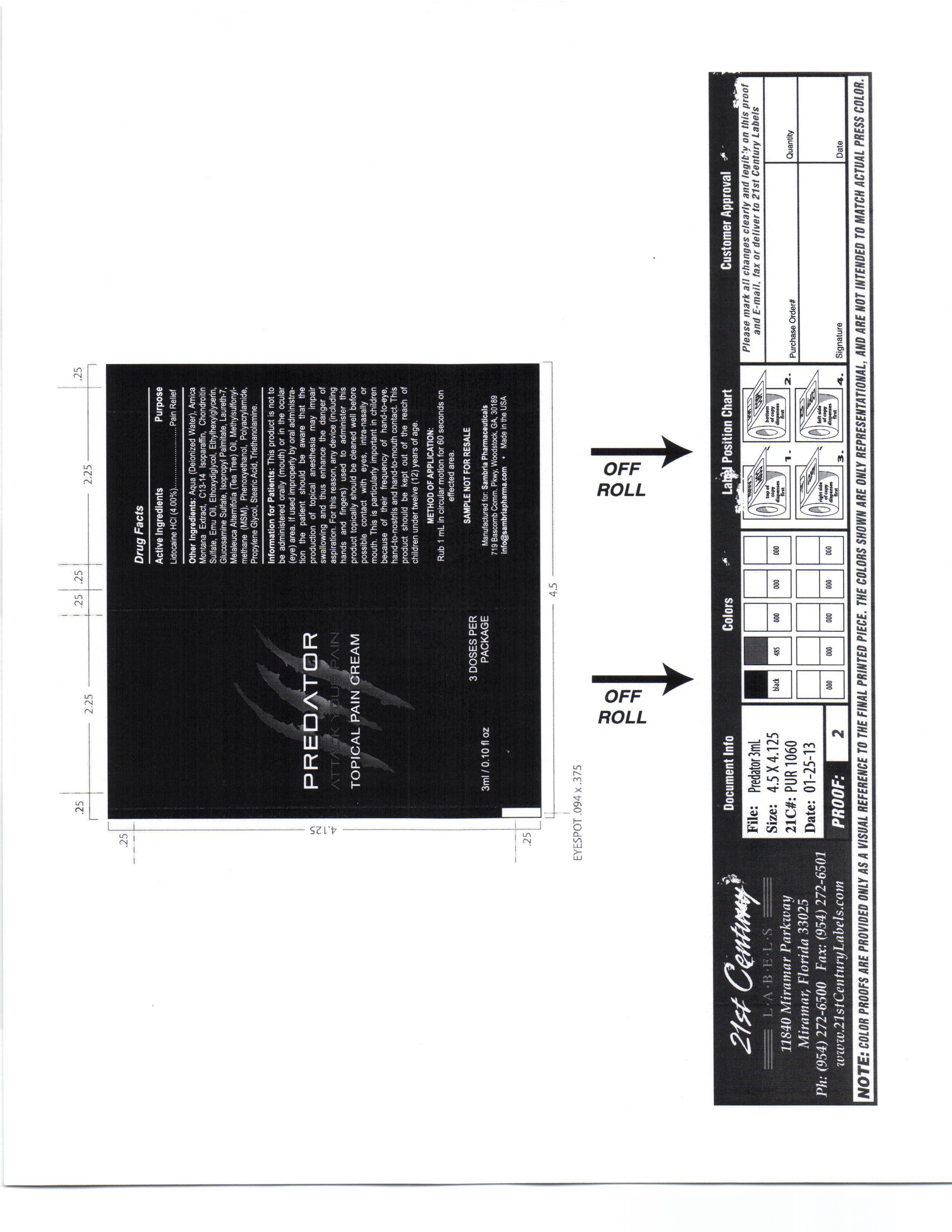
PREDATOR- lidocaine hcl cream
Predator by
Drug Labeling and Warnings
Predator by is a Otc medication manufactured, distributed, or labeled by Sambria Pharmaceuticals, LLC, Pure Source. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Information for Patients
This product is not to be administered orally (mouth) or in the ocular (eye) area.
If used improperly by oral administration the patient should be aware that the production of topical anesthesia may impair swallowing and thus enhance the danger of aspiration. For this reason, any device (including hands and fingers) used to administer this product topically should be cleaned well before possible contact with eyes, intra-nasaly or mouth.
- active ingredients
-
Other ingredients
Aqua, Amica Montana Extract, C13-14 Isoparafin, Chondrotin Sulfate, Emu Oil, Ethoxydiglycol, Ethylhexylglycerin, Glucosamine Sulfate, Isopropyl Palmitate, Laureth 7, Melaleuca Alternifoil (Tea Tree) oil, Methylsulfonylmethana (MSM), Phenoxyethanol, Polyacrylamide, Propylene Glycol, Stearic Acid, Triethanolamine
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- Method of Application
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREDATOR
lidocaine hcl creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54723-150 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 400 mg in 1 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) EMU OIL (UNII: 344821WD61) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) LAURETH-7 (UNII: Z95S6G8201) TEA TREE OIL (UNII: VIF565UC2G) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHANOLAMINE BENZOATE (UNII: M3EN4GC19W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54723-150-03 400 mg in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/11/2013 Labeler - Sambria Pharmaceuticals, LLC (078676259) Establishment Name Address ID/FEI Business Operations Pure Source 969241041 manufacture(54723-150)
Trademark Results [Predator]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.