SPF 30 CONCEALER- spf 30 concealer cream
Vrea LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Outer Carton
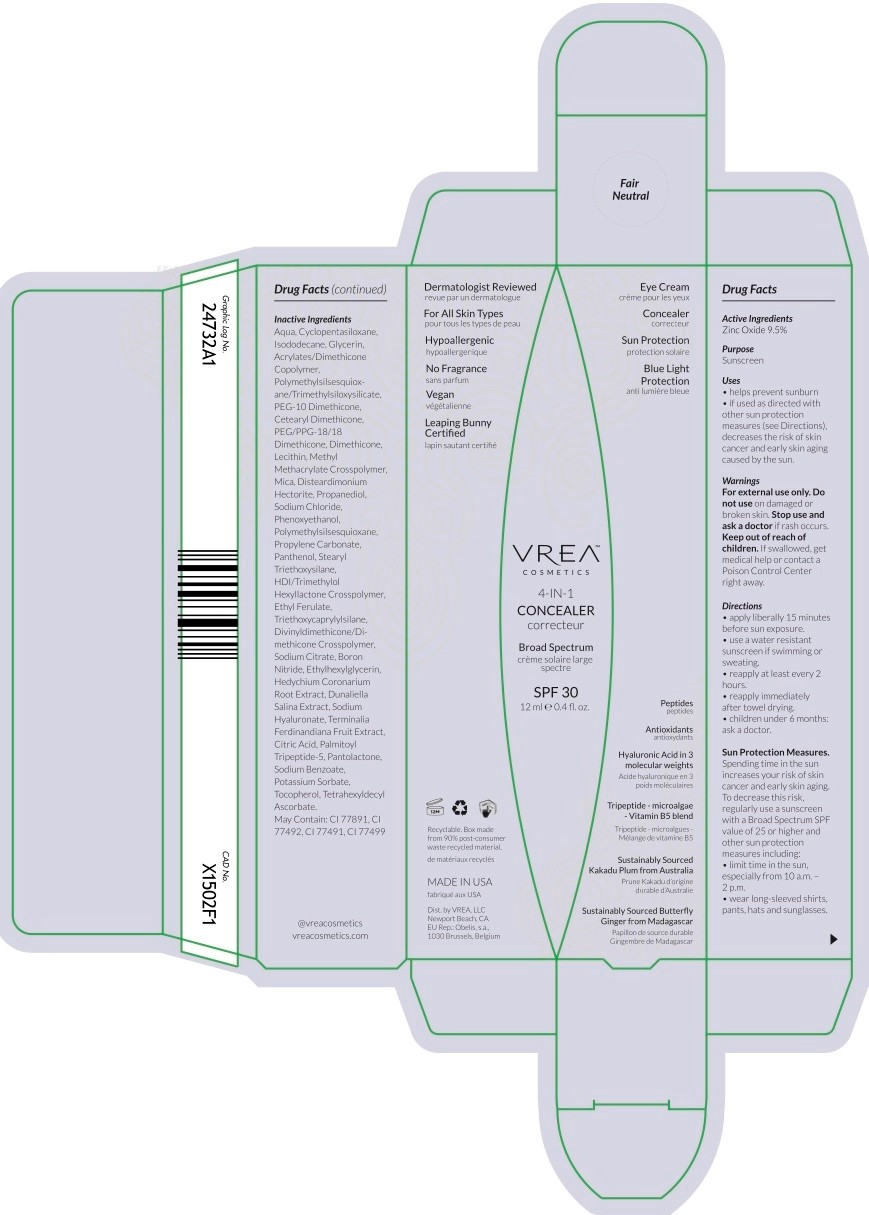
Principal Display Panel
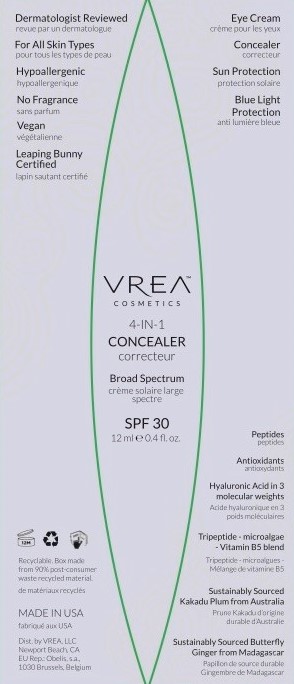
SPF 30 CONCEALER
spf 30 concealer cream |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 81854-001 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) | ZINC OXIDE | 9.3154 g in 100 g |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) | 0.108665 g in 100 g |
| PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) | 3.1 g in 100 g |
| PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) | 2.0705 g in 100 g |
| METHYL METHACRYLATE (UNII: 196OC77688) | 1.67 g in 100 g |
| ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) | 0.21 g in 100 g |
| BORON NITRIDE (UNII: 2U4T60A6YD) | 0.185 g in 100 g |
| STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) | 0.2499 g in 100 g |
| DUNALIELLA SALINA (UNII: F4O1DKI9A6) | 0.0309 g in 100 g |
| TITANIUM DIOXIDE (UNII: 15FIX9V2JP) | 12.40115 g in 100 g |
| ISODODECANE (UNII: A8289P68Y2) | 7.893 g in 100 g |
| DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) | 0.9288 g in 100 g |
| POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) | 0.606 g in 100 g |
| TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) | 0.2973 g in 100 g |
| ETHYL FERULATE (UNII: 5B8915UELW) | 0.2973 g in 100 g |
| HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) | 0.062 g in 100 g |
| PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) | 0.005665 g in 100 g |
| PANTOLACTONE, (+/-)- (UNII: IID733HD2M) | 0.005665 g in 100 g |
| TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) | 0.003 g in 100 g |
| DIMETHICONE 100 (UNII: RO266O364U) | 2.0295 g in 100 g |
| LECITHIN, SOYBEAN (UNII: 1DI56QDM62) | 2.01 g in 100 g |
| MICA (UNII: V8A1AW0880) | 1.665 g in 100 g |
| PROPYLENE CARBONATE (UNII: 8D08K3S51E) | 0.258 g in 100 g |
| ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) | 0.005665 g in 100 g |
| POTASSIUM SORBATE (UNII: 1VPU26JZZ4) | 0.005665 g in 100 g |
| GLYCERIN (UNII: PDC6A3C0OX) | 4.117 g in 100 g |
| PROPANEDIOL (UNII: 5965N8W85T) | 1.29 g in 100 g |
| SODIUM CHLORIDE (UNII: 451W47IQ8X) | 1.26 g in 100 g |
| PHENOXYETHANOL (UNII: HIE492ZZ3T) | 0.9579 g in 100 g |
| PANTHENOL (UNII: WV9CM0O67Z) | 0.3605 g in 100 g |
| WATER (UNII: 059QF0KO0R) | 18.795145 g in 100 g |
|
| Product Characteristics |
| Color | brown (Medium Neutral) , brown (Light) , brown (Deep Honey) , brown (Light Neutral) , brown (Rich Neutral) , brown (Fair Neutral) | Score | |
| Shape | | Size | |
| Flavor | | Imprint Code | |
| Contains | |
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 81854-001-01 | 1 in 1 CARTON | 06/01/2021 | |
| 1 | | 14 g in 1 TUBE; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part352 | 06/01/2021 | |
|
| Labeler - Vrea LLC.
(057455597)
|
| Registrant - Vrea LLC. (057455597) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| New Look Cosmetics | | 080801719 | manufacture(81854-001) |



