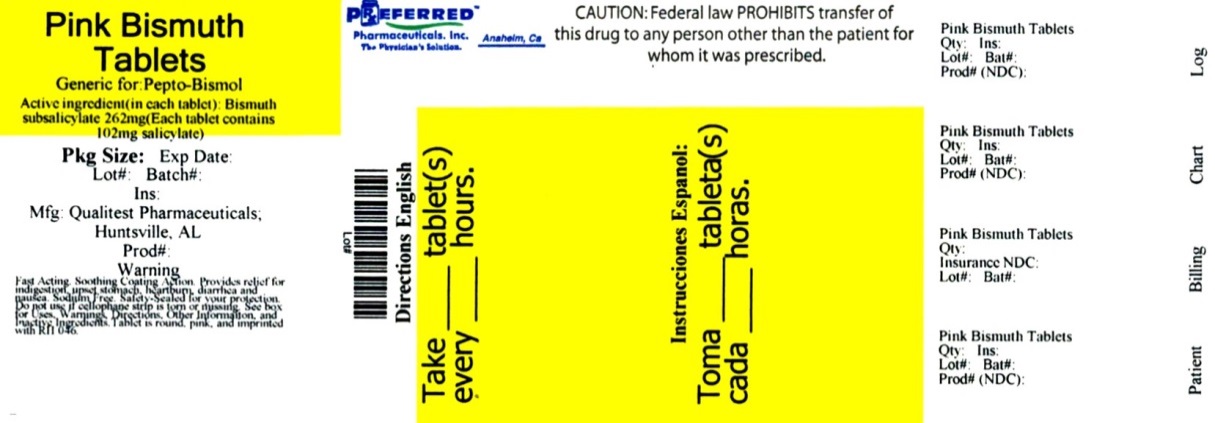
Pink Bismuth by Preferred Pharmaceuticals, Inc Drug label
Pink Bismuth by
Drug Labeling and Warnings
Pink Bismuth by is a Otc medication manufactured, distributed, or labeled by Preferred Pharmaceuticals, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PINK BISMUTH- bismuth subsalicylate tablet, chewable
Preferred Pharmaceuticals, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug label
Uses
- Controls diarrhea
-
relieves heartburn, indigestion, nasea and upset stomach without constipating
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea or vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert
Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
Do not use if you have
- an ulcer
- a bleeding problem
- bloody or black stool
Ask a doctor before use if you have
- fever
- mucus in the stool
Ask a doctor or pharmacist before use if you are taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
Stop use and ask a doctor if
- symptoms get worse
- ringing in the ears or loss of hearing occurs
- diarrhea lasts more than 2 days
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Adults and Children 12 years and over: 525mg (2 tablets) every 1/2 to 1 hour or 1050mg (4 tablets) every hours as needed. Do not exceed 4200mg (16 tablets) in 24 hours. Use until diarrhea stops but not more than 2 days. Children under 12 years ask a doctor
Other information:
- Save carton for full directions and warnings
- Store at controlled room temperature 59°- 86°F (°15 - 30°C)
- Do not use is cellophane unit is torn, cut or open.
- Phenylketonurics: Contains phenylalanine 1.1mg per tablet.
-
Calcium content per tablet, 73mg
| PINK BISMUTH
bismuth subsalicylate tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Preferred Pharmaceuticals, Inc (791119022) |
| Registrant - Preferred Pharmaceuticals, Inc (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals, Inc | 791119022 | REPACK(68788-0190) | |