Afrin ® Severe Congestion
Afrin by
Drug Labeling and Warnings
Afrin by is a Otc medication manufactured, distributed, or labeled by Delpharm Montreal Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AFRIN SEVERE CONGESTION- oxymetazoline hydrochloride spray
Delpharm Montreal Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Afrin
®
Severe Congestion
Uses
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily relieves sinus congestion and pressure
- shrinks swollen nasal membranes so you can breathe more freely
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: ask a doctor.
To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use.
Other information
- store between 20° to 25°C (68° to 77°F)
- retain carton for future reference on full labeling
Inactive ingredients
benzalkonium chloride solution, benzyl alcohol, camphor, edetate disodium, eucalyptol, menthol, polysorbate 80, propylene glycol, purified water, sodium phosphate dibasic, sodium phosphate monobasic
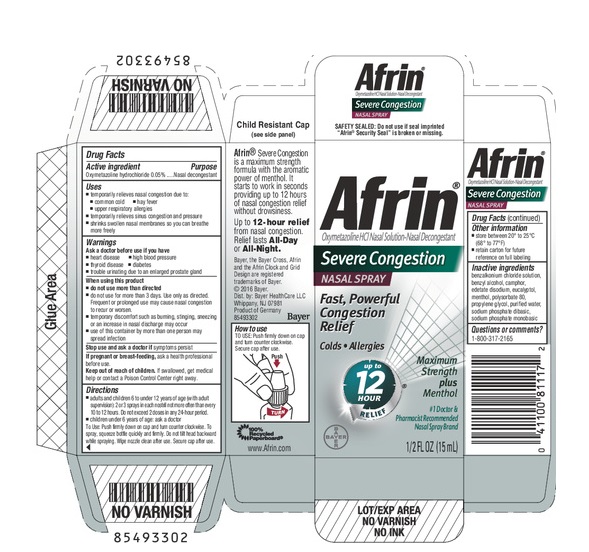
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
Afrin®
Oxymetazoline HCl Nasal Solution-Nasal Decongestant
Severe Congestion
NASAL SPRAY
Fast, Powerful
Congestion
Relief
Colds Allergies
up to 12
Hour
Relief
Maximum
Strength
plus Menthol
#1 Doctor & Pharmacist
Recommended Nasal Brand
BAYER
1/2 FL OZ (15 mL)
| AFRIN
SEVERE CONGESTION
oxymetazoline hydrochloride spray |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Delpharm Montreal Inc. (203565379) |
Trademark Results [Afrin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AFRIN 97713514 not registered Live/Pending |
Turkana Food Inc 2022-12-12 |
 AFRIN 76294680 2547372 Live/Registered |
BAYER HEALTHCARE LLC 2001-08-02 |
 AFRIN 75774350 2420923 Dead/Cancelled |
SCHERING CORPORATION 1999-08-12 |
 AFRIN 75451774 2291563 Dead/Cancelled |
SCHERING CORPORATION 1998-03-18 |
 AFRIN 74455066 1862221 Dead/Cancelled |
Schering Corporation 1993-11-08 |
 AFRIN 74455065 1862220 Dead/Cancelled |
Schering Corporation 1993-11-08 |
 AFRIN 74072001 1709138 Live/Registered |
Schering Corporation 1990-06-22 |
 AFRIN 73083784 1052754 Live/Registered |
SCHERING CORPORATION 1976-04-13 |
 AFRIN 72191218 0789309 Dead/Expired |
SCHERING CORPORATION 1964-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.