Children's Allergy Relief 5 mL vial/ampoule Correct Dose
Childrens Allergy Relief by
Drug Labeling and Warnings
Childrens Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Plastikon Healthcare, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CHILDRENS ALLERGY RELIEF- diphenhydramine hydrochloride liquid
Plastikon Healthcare, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Children's Allergy Relief 5 mL vial/ampoule
Correct Dose
Diphenhydramine HCl 12.5 mg / 5 mL Vial/Ampoule

Single use measurements
for easy accurate dosing
*compare to the active ingredients in children's Benadryl® allergy liquid
CORRECTDOSE
Precise - Portable - Single-Use
Children's
ALLERGY
RELIEF
Diphenhydramine HCl
Oral solution Antihistamine
Cherry
12
Pre-Filled
Single Use
Doses
Relieves
Sneezing, Running Nose,
Itchy & Watery Eyes,
Itchy Throat
12.04 FL. OZ (60 mL)
12 - 5 mL Individual Doses
Diphenhydramine HCl 12.5 mg/ 5 mL
Warnings
Do not use
to make a child sleepy
with any other product containing diphenhydramine, even one used on skin
if hypersensitive to diphenhydramine HCl and other similar antihistamines
___________________________________________________________________
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis
a sodium restricted diet
___________________________________________________________________
Ask a doctor or pharmacist before use if
the child is taking tranquilizers or sedatives
___________________________________________________________________
When using this product
marked drowsiness may occur
sedatives, and tranquilizers may increase drowsiness
be careful when driving a motor vehicle or operating machinery
excitability may occur, especially in children
Diphenhydramine HCl 12.5 mg/ 5 mL
Inactive ingredients cherry flavor, citric acid, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucralose
Diphenhydramine HCl 12.5 mg / 5 mL
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose sneezing itchy, watery eyes itchy throat
Diphenhydramine HCl 12.5 mg/ 5 mL
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Diphenhydramine HCl 12.5 mg/ 5 mL
Active ingredient (in each 5 mL unit)
Diphenhydramine HCl USP 12.5 mg
Diphenhydramine HCl 12.5 mg/ 5 mL
each 5 mL contains: sodium 15 mg
- store at 20-25°C (68-77°F)
- do not use if foil seal is opened/damaged
- see bottom of bag for lot number and expiration date
- protect from excessive moisure
- sugar free, dye free, alcohol free
Children's Allergy Relief Diphenhydramine 12.5 mg/5 mL Single Use Pouch
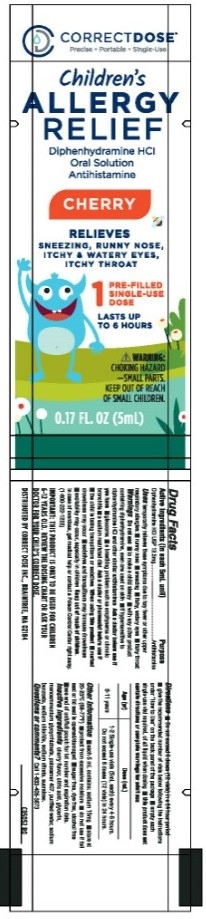
CORRECTDOSE
Precise - Portable - Single-Use
Children's
ALLERGY
RELIEF
Diphenhydramine HCl
Oral Solution
Antihistamine
Cherry
Relieves
Sneezing, Runny Nose,
Itchy & Watery Eyes,
Itchy Throat
1
Pre-Filled
Single-Use
Dose
Lasts up to
6 hours
WARNING
Choking Hazard
- Small parts
Keep out of reach
of small children
0.17 FL. OZ (5 mL)
Diphenhydramine HCl 12.5 mg/ 5 mL
Directions
- Do not exceed 6 doses (12 vials) in a 24 hour period
- give the recommended number of vials below following the instructions under How to Use on the back panel
- empty each single-use vial required, of all liquid when dosing
- this product does not contain directions or complete warnings for adult use
|
Age (yr) |
Dose (mL) |
|
6-11 years |
1-2 Single-use vials (5 mL each) every 4-6 hours Do not exceed 6 doses (12 vials) in a 24 hours |
| CHILDRENS ALLERGY RELIEF
diphenhydramine hydrochloride liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Plastikon Healthcare, LLC (041717941) |
| Registrant - Plastikon Healthcare, LLC (041717941) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Plastikon Healthcare, LLC | 041717941 | manufacture(62320-302) | |