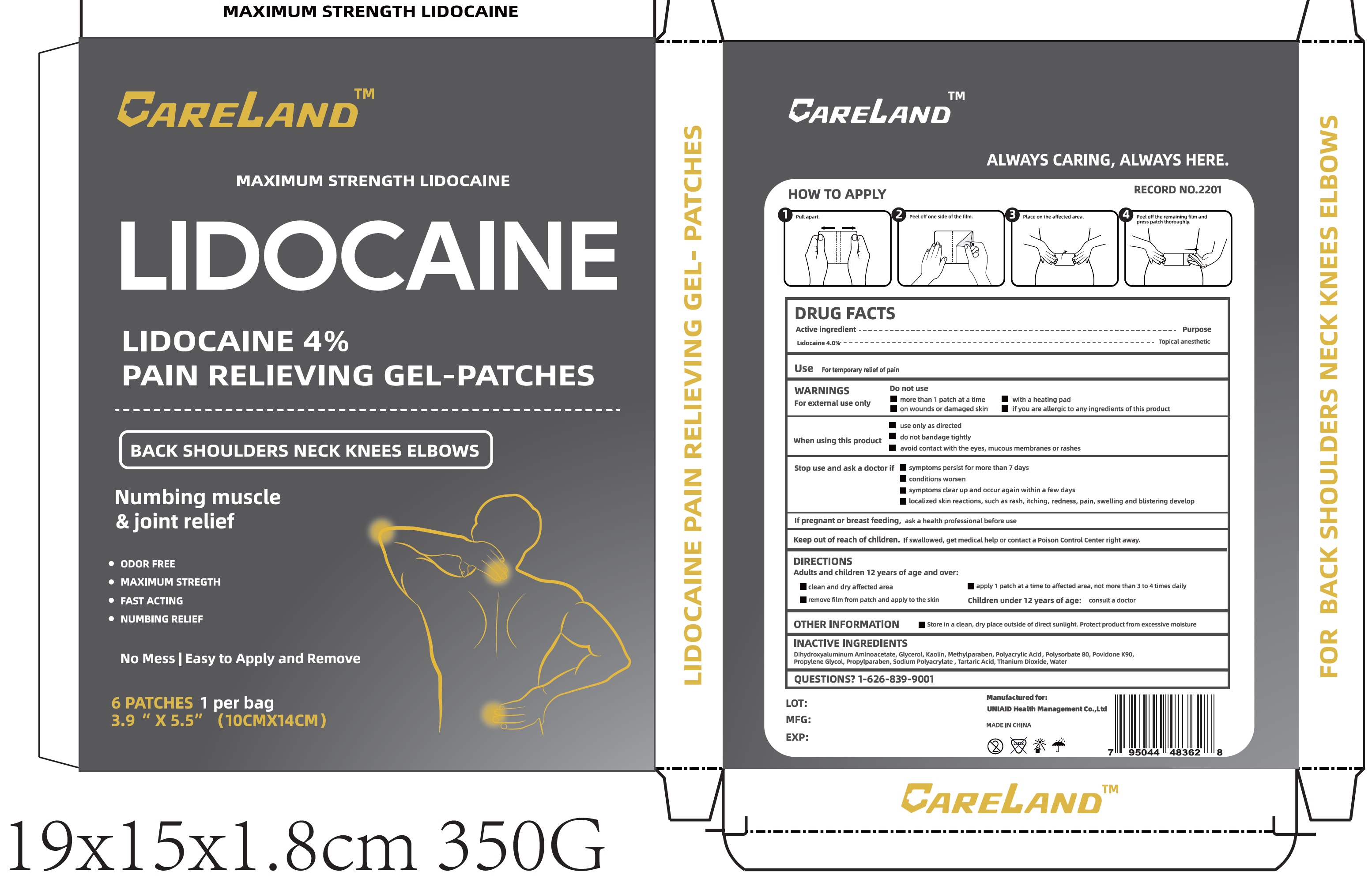
PAIN RELIEVING GEL-PATCHES
PAIN RELIEVING by
Drug Labeling and Warnings
PAIN RELIEVING by is a Otc medication manufactured, distributed, or labeled by UNIAID Health Management (Suzhou) Co.,Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEVING- lidocaine patch
UNIAID Health Management (Suzhou) Co.,Ltd
----------
PAIN RELIEVING GEL-PATCHES
WARNINGS
For external use only
Do not use
- more than 1 patch at a time
- with a heating pad
- on wounds or damaged skin
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- do not bandage tightly
- avoid contact with the eyes, mucous membranes or rashes
DIRECTIONS
Adults and children 12 years of age and over:
- clean and dry affected area
- apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- remove film from patch and apply to the skin
Children under 12 years of age: consult a doctor
OTHER INFORMATION
- Store in a clean, dry place outside of direct sunlight, Protect product from excessive moisture
| PAIN RELIEVING
lidocaine patch |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - UNIAID Health Management (Suzhou) Co.,Ltd (412839778) |
Revised: 12/2025
Document Id: 44f15a79-a5b0-4047-e063-6394a90aefd5
Set id: c1203d42-7d31-4ae8-90ee-ac8c85344737
Version: 3
Effective Time: 20251201
U
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.