LXR CoFix Povidone-Iodine Sanitizer
CoFix RX by
Drug Labeling and Warnings
CoFix RX by is a Otc medication manufactured, distributed, or labeled by LXR Biotech LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
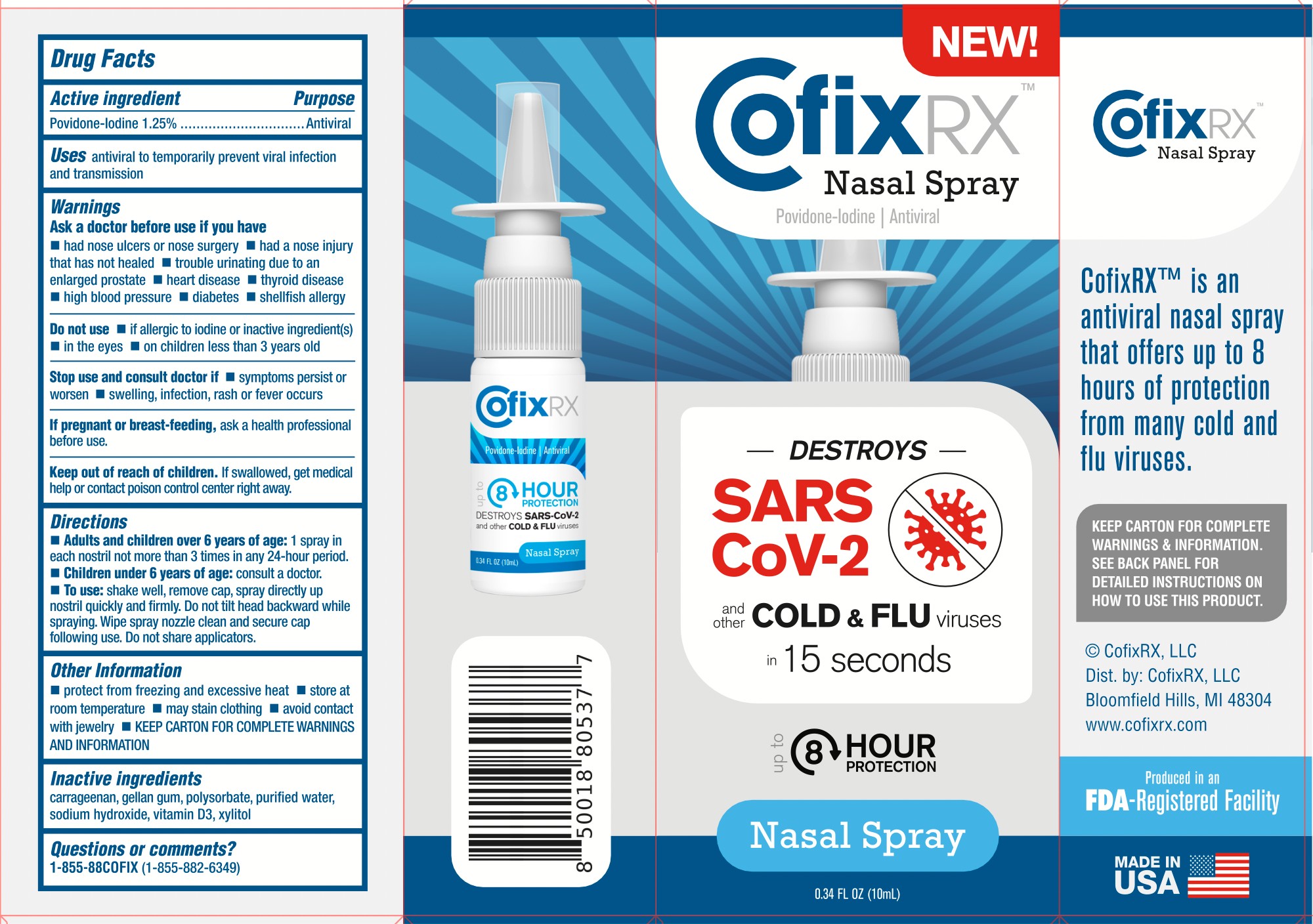
COFIX RX- povidone-iodine antiviral nasal spray liquid
LXR Biotech LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LXR CoFix Povidone-Iodine Sanitizer
Warnings
Ask a doctor before use if you have
■ had nose ulcers or nose surgery ■ had a nose injury that has not healed ■ trouble urinating due to an enlarged prostate ■ heart disease ■ thyroid disease ■ high blood pressure ■ diabetes ■ shellfish allergy
Do not use
■ if allergic to iodine or inactive ingredient(s) ■ in the eyes ■ on children less than 3 years old
Stop use and ask a doctor if symptoms persist or worsen ■ swelling, infection, rash, or fever occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ Adults and children over 6 years of age: 1 spray in each nostril not more than 3 times in any 24-hour period
■ Children under 6 years of age: consult a doctor
■ To use shake well, remove cap, spray directly up nostril quickly and firmly. Do not tilt head backward while spraying. Wipe spray nozzle clean and secure cap following use. Do not share applicators.
Other information
protect from freezing and excessive heat ■ store at room temperature ■ may stain clothing ■ avoid contact with jewelry ■ Keep the carton for complete warning and information
| COFIX RX
povidone-iodine antiviral nasal spray liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - LXR Biotech LLC (117520926) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LXR Biotech LLC | 968357405 | manufacture(78299-211) | |
Trademark Results [CoFix RX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COFIX RX 90337951 not registered Live/Pending |
CoFix-RX, LLC 2020-11-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 10 mL NDC:
10 mL NDC: