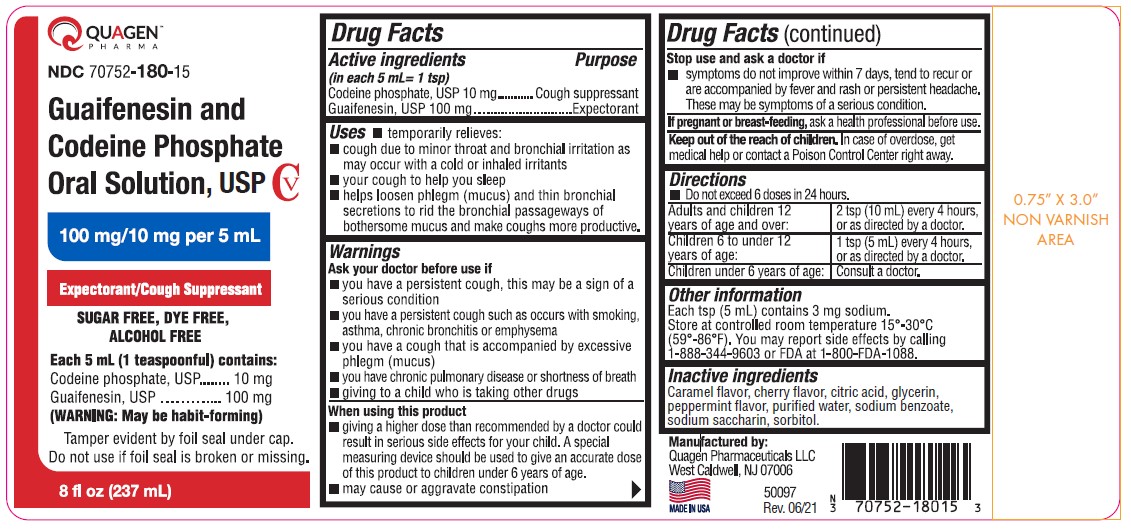
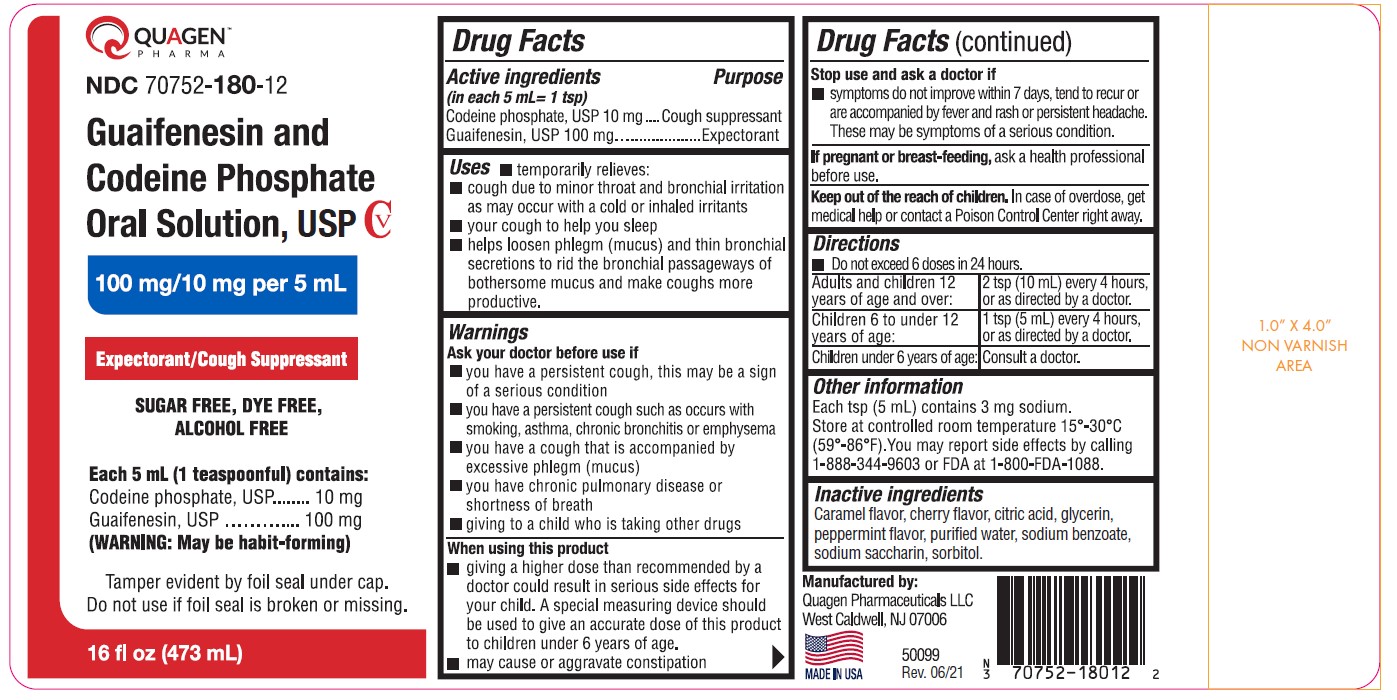
Guaifenesin and Codeine Phosphate Oral Solution, USP CV100 mg/10 mg per 5 mL SUGAR FREE, DYE FREE, ALCOHOL FREE
GUAIFENESIN and CODEINE PHOSPHATE by
Drug Labeling and Warnings
GUAIFENESIN and CODEINE PHOSPHATE by is a Otc medication manufactured, distributed, or labeled by QUAGEN PHARMACEUTICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GUAIFENESIN AND CODEINE PHOSPHATE- guaifenesin and codeine phosphate solution
QUAGEN PHARMACEUTICALS LLC
----------
Guaifenesin and Codeine Phosphate Oral Solution, USP CV
100 mg/10 mg per 5 mL
SUGAR FREE, DYE FREE, ALCOHOL FREE
Uses
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- your cough to help you sleep
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive.
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
Stop use and ask a doctor if
- symptoms do not improve within 7 days, tend to recur or are accompanied by fever and rash or persistent headache. These may be symptoms of a serious condition.
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Do not exceed 6 doses in 24 hours.
|
Adults and children 12 years of age and over: |
2 tsp (10 mL) every 4 hours, |
|
Children 6 to under 12 years of age: |
1 tsp (5 mL) every 4 hours, or as directed by a doctor. |
|
Children under 6 years of age: |
Consult a doctor. |
Other information
Each tsp (5 mL) contains 3 mg sodium.
Store at controlled room temperature 15°-30°C (59°-86°F). You may report side effects by calling 1-888-344-9603 or FDA at 1-800-FDA-1088.
Inactive ingredients
Caramel flavor, cherry flavor, citric acid, glycerin, peppermint flavor, purified water, sodium benzoate, sodium saccharin, sorbitol.
| GUAIFENESIN AND CODEINE PHOSPHATE
guaifenesin and codeine phosphate solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - QUAGEN PHARMACEUTICALS LLC (073645339) |
| Registrant - QUAGEN PHARMACEUTICALS LLC (073645339) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| QUAGEN PHARMACEUTICALS LLC | 080281331 | manufacture(70752-180) , pack(70752-180) | |